Figure 3.
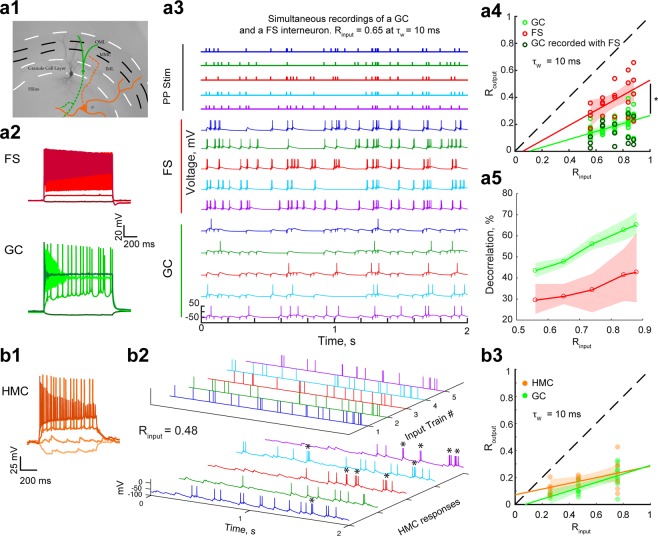
Different levels of temporal pattern separation in different DG cell types. (a1) Picture of a recorded FS filled with biocytin (black). In the case of simultaneous recordings, the recorded GCs were close to the FS, as depicted by the schematic in green. In different experiments, we recorded from putative hilar mossy cells (HMC, orange). Full lines represent dendrites, dashed lines axons. (a2-3) Example of a simultaneous whole-cell recording of a GC and a neighboring FS. (a2) Simultaneous membrane potential recordings (baseline at −60 mV) of a FS and a GC to the same set of current steps (−25, 100, 500 and 1000 pA). (a3) Simultaneous current-clamp recordings of the same FS and GC as in a2 in response to the five input traces of an input set with Rinput = 0.65. Simultaneous input and output trains have the same color. (a4) Routput versus Rinput for FSs and GCs. Data points correspond to recording sets: 20 for FS (red, 4 cells, 4 per input set), and 61 for GC (green, with a darker shade open circle when simultaneously recorded with a FS, 13 cells, 11–13 per input set). All GC recordings done at the same input correlations as FS recordings were used for an unpaired comparison showing that FS exhibit less spiketrain decorrelation than GCs: ANCOVA: p < 0.0001, 95% confidence bounds around the linear fits shown as shaded areas; two-way ANOVA: input sets: p = 0.0016, cell-types: p < 0.0001, interaction: p = 0.72. Post-hoc T-tests with Bonferroni corrections for 5 comparison groups: all p < 0.05 (for decreasing Rinput, p = 0.0307, 0.0181, 0.0007, 0.0001, 0.0122). (a5) Effective decorrelation (Rinput – Routput) for FS and GC. Shaded areas represent the 95% confidence interval of a bootstrap test comparing the mean decorrelation of both celltypes: GCs exhibit significantly more pattern separation than FS for all input sets. (a4–5) Note that when comparing only the simultaneous GC and FS recordings, we found a similarly significant difference between celltypes. (b1) Membrane potential of a hilar neuron in response to current steps (−100, 0, 100, 400 pA; baseline at −70 mV), showing a spontaneous barrage of EPSPs, regular spiking, and a lack of large after-hyperpolarization, all typical features of HMCs (Larimer & Strowbridge, 2008). (b2) Current-clamp recordings of same HMC in response to a set of five input trains (Rinput = 0.48, τw = 10 ms). HMCs fire occasional bursts of spikes (marked by asterisks) in response to a single input, which was not seen in GCs. (b3) Routput versus Rinput for HMCs and GCs. Data points correspond to recording sets: 18 for HMC (orange, 11 cells, 5–7 per input set), and 22 for GC (green, 11 cells, 4–10 per input set). An unpaired comparison suggests that HMCs and GCs show only slight differences in pattern separation measured at τw = 10 ms: ANCOVA: p = 0.15, 95% confidence bounds around the linear fits shown as shaded areas; two-way ANOVA: input sets: p = 0.0004, cell-types: p = 0.074, interaction: p = 0.57. Post-hoc T-tests with Bonferroni corrections for three comparison groups (for decreasing Rinput): p = 1, 0.05, 0.21.