Figure 4.
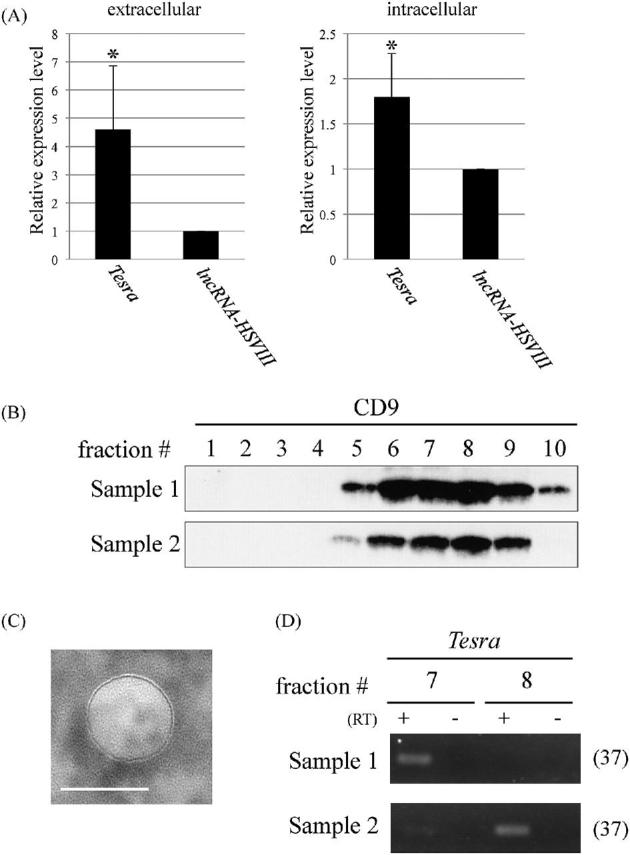
Extracellular localization of Tesra. (A) Expression of Tesra in the extracellular fraction (left) and germ cells (right) from adult mouse testes. The extracellular fraction was collected by immersing tissue pieces of adult testes in a cell culture medium, and total RNAs were purified. Germ cells were also investigated to measure the intracellular level. Complementary DNAs were synthesized with a random hexamer for the extracellular fraction and with the oligo(dT) primer for germ cells, and real-time PCR was performed to amplify Tesra and lncRNA-HSVIII. Since the amplification efficiencies were not so different, the expression levels were directly compared by setting the value of lncRNA-HSIII to 1.0. The data are presented as means ± SD from three independent experiments and were analyzed by the Student t test. *P < 0.05 relative to the control. (B) Western blot analysis of fractions collected by size exclusion chromatography. The extracellular fraction was applied to the column to separate EVs, and 10 fractions were eluted. A portion of each fraction was subjected to western blot analysis to detect the CD9 protein, a widely used exosome marker. We performed duplicate experiments, and both results are shown. Intense signals were detected in fractions #6-#9 of both samples. (C) Electron microscopic observation of an EV. A representative photograph of an EV in a fraction that was obtained by size exclusion chromatography and was positive for CD9. Similar vesicles were observed on the mesh. A scale bar, 100 nm. (D) RT-PCR with exosome fractions. Total RNAs were purified from fractions #7 and #8 of both samples in (B), and cDNAs were synthesized with a random hexamer. Tesra signals were detected by 37 cycles of PCR and agarose gel electrophoresis. In sample 1, the signal was observed in fraction #7, and in sample 2, both lanes showed the band, although the signal intensity was much higher in fraction #8.
