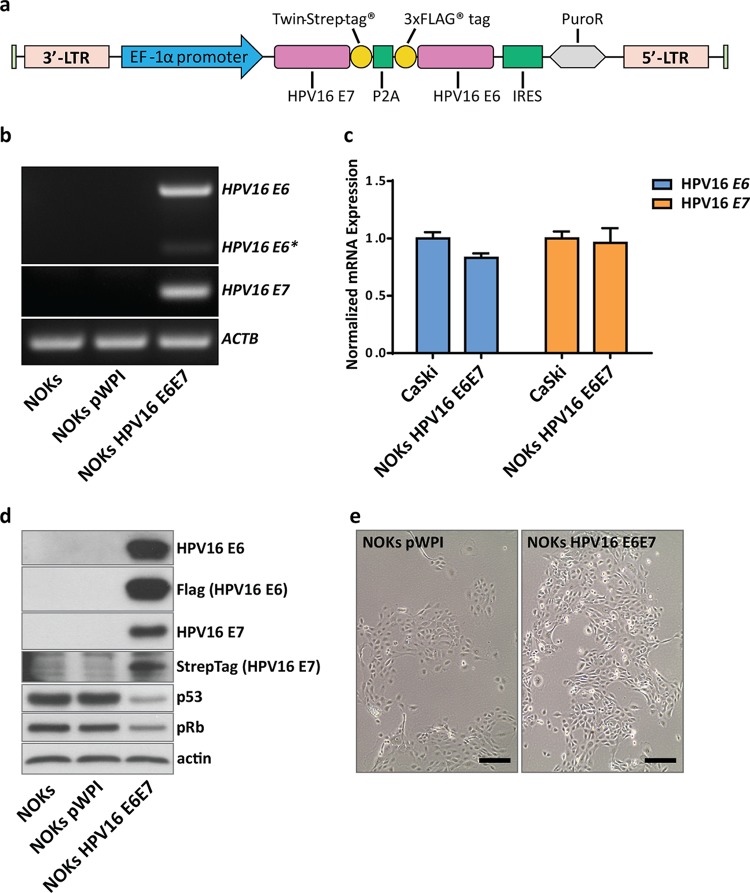
FIG 1.
Establishment of a keratinocyte cell line expressing HPV16 E6 and E7. (a) Schematic representation of the vector construct used to stably express E6 and E7 in NOKs. Strep-tagged E7 and Flag-tagged E6, separated by a P2A sequence, were cloned into the pWPI vector for lentiviral packaging. NOK cells were subsequently transduced and selected with puromycin. Pools of oncogene-transduced or empty vector control cells were used for all further studies. 3′-LTR, 3′ long terminal repeat; IRES, internal ribosome entry site. (b) Semiquantitative PCR of transduced NOKs. RNA of untransduced (NOKs), NOKs with an empty vector control (NOKs pWPI), and NOKs with oncogene-transduced cells (NOKs HPV16 E6E7) was reverse transcribed, and PCR was performed with primers for HPV16 E6/E6* and E7. ACTB served as an internal control. (c) Quantitative PCR comparing relative transcript levels of E6 and E7 from NOKs HPV16 E6E7 with the HPV16-positive cancer cell line CaSki. Transcript levels were normalized to those of TOP1 as a housekeeping gene. (d) Western blot analyses of untransduced and transduced NOKs. Protein lysates (30 µg) were analyzed for the presence of HPV16 E6 and E7 using oncoprotein-specific antibodies as well as anti-Flag (E6) antibody or streptavidin-HRP (E7). Levels of p53 and pRb were analyzed to confirm the functionality of the viral proteins. Actin served as a loading control. (e) Light microscopic image of transduced cells prior to RNA extraction. Scale bar = 200 µM.