Figure 2.
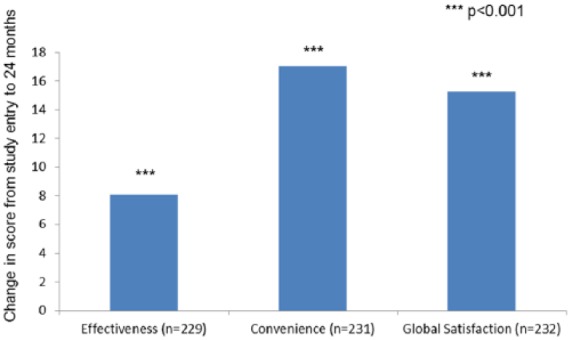
TSQM-9: Changes in effectiveness, convenience, and global satisfaction scores from study entry to 24 months for patients receiving teriflunomide who discontinued previous DMT within 6 months of study entry.
***p < 0.001.
DMT, disease-modifying therapy; TSQM-9, Treatment Satisfaction Questionnaire.
