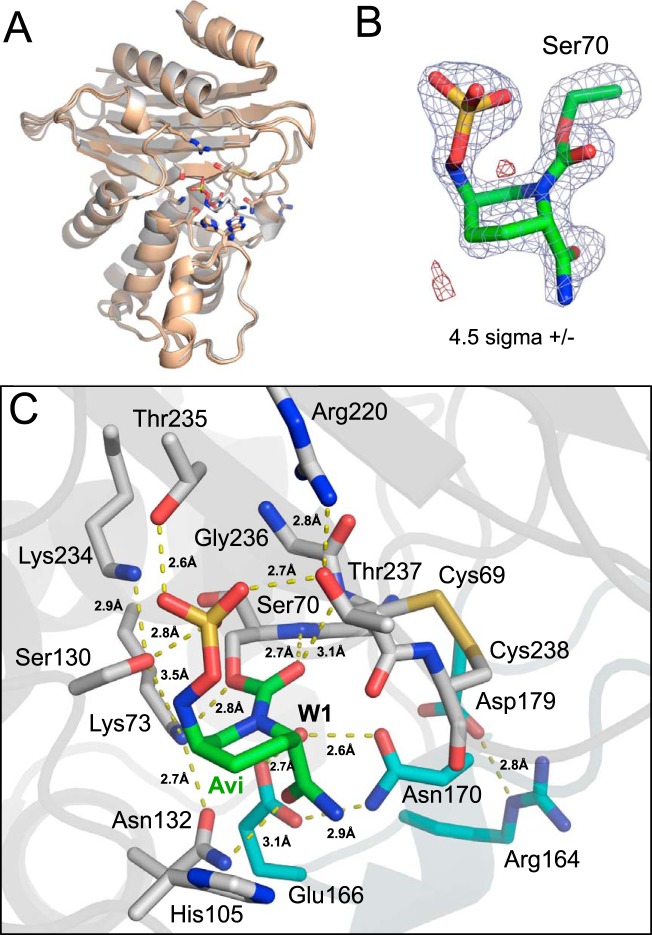
FIG 4.
X-ray structure of VCC-1 bound to avibactam. (A) Superposition of the avibactam-bound VCC-1 structure with the 4 copies of VCC-1 from the asymmetric unit of the native X-ray structure. Avibactam binding does not significantly alter the active site or overall conformation of the β-lactamase (RMSD < 0.8 Å). (B) Electron density (|Fobs| − |Fcalc| omit map contoured at 4.5 σ) defining avibactam (green carbons) covalently bound to Ser70 (gray carbons) of VCC-1. Positive density is blue, and negative density is red. (C) Avibactam (Avi; green carbon atoms) covalently bound to Ser70 within the VCC-1 active site. VCC-1 carbon atoms are gray, with the exception of residues within the omega loop, which are colored cyan. Dashed yellow lines represent hydrogen-bonding interactions, with their respective distances indicated.