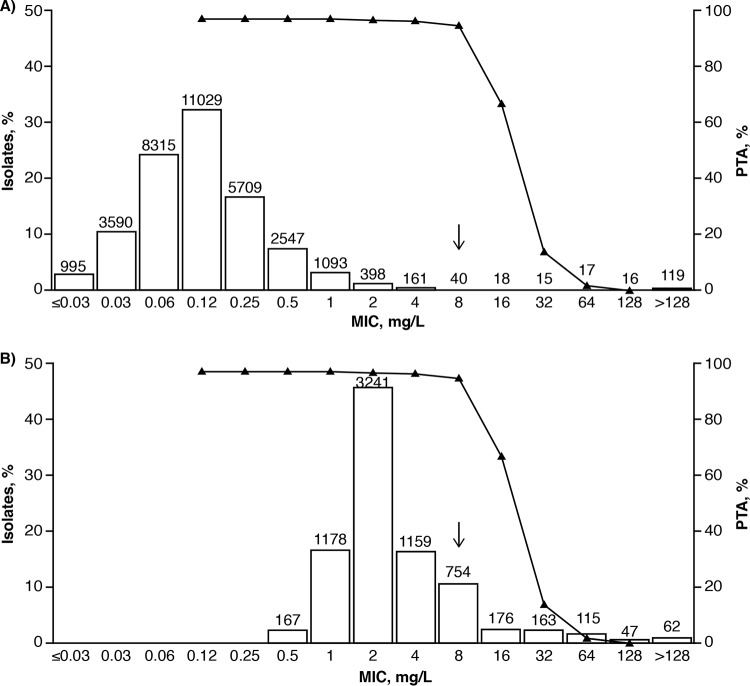
FIG 1.
Joint PTA for patients with cIAI receiving ceftazidime-avibactam 2,000 + 500 mg q8h plotted as a function of ceftazidime-avibactam MIC overlaying the ceftazidime-avibactam MIC distributions against Enterobacteriaceae (n = 34,062) (A) and Pseudomonas aeruginosa (n = 7,062) (B) from the INFORM global surveillance study (2012 to 2014). The figure is from Nichols et al. (89). Joint PTA is defined as simultaneous attainment of 50% fT>MIC of ceftazidime-avibactam for ceftazidime and 50% fT>CT of 1 mg/liter for avibactam, with both targets having to be achieved for a simulated patient to be categorized as achieving the joint target. The joint PTA was calculated using iteration 4 of the population PK models (see Table 2). Ceftazidime-avibactam MIC distributions were obtained from the INFORM 2012–2014 global surveillance study (7, 11, 60, 88). Values above the bars are the numbers of isolates tested at each MIC. The arrows show the position of the approved ceftazidime-avibactam susceptible clinical breakpoint of MIC ≤8 mg/liter.