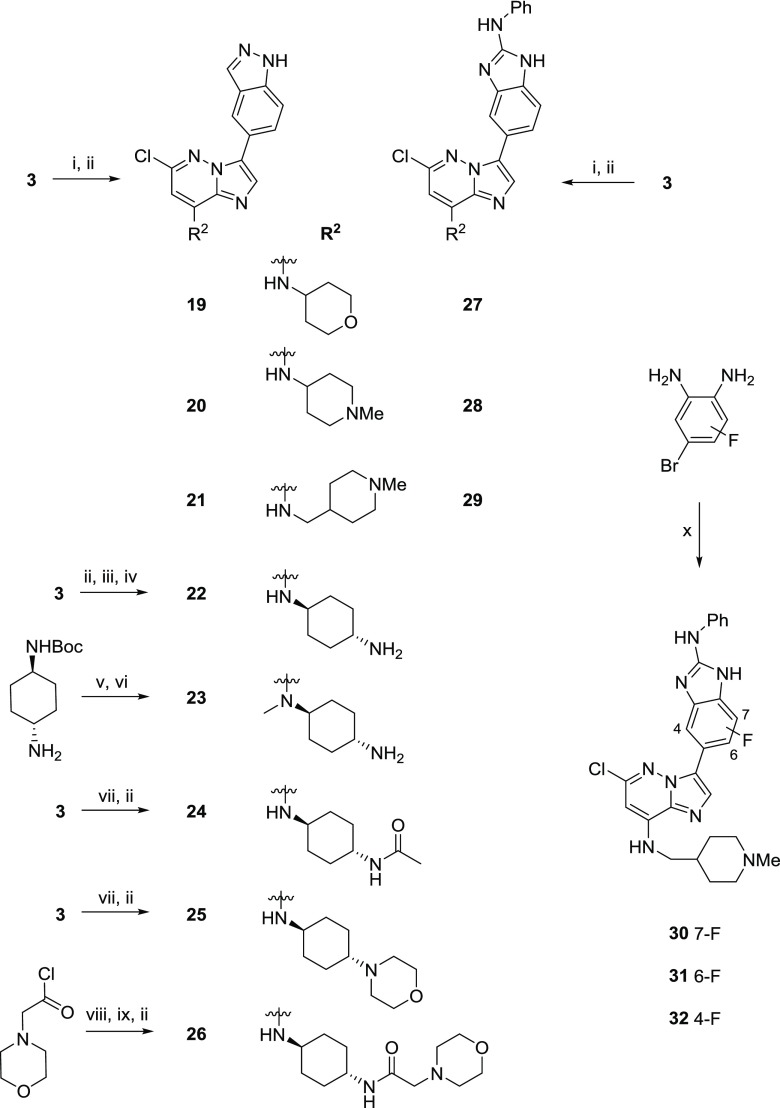
Scheme 2. Syntheses of Compounds 19–32.
Reagents and conditions: (i) amine, THF, rt, 16 h, 60–97%; (ii) heteroaryl boronate, Pd(OAc)2, 1,1′-bis(di-tert-butylphosphino)ferrocene, 2 M Na2CO3, 1,4-dioxane, 130 °C, 16 h, 4–55%; (iii) tert-butyl ((1r,4r)-4-aminocyclohexyl)carbamate, THF, 50 °C, 16 h, quantitative; (iv) HCl, 1,4-dioxane, rt, 2 h, 99%; (v) (a) LiAlH4, THF, 0 °C to reflux, 4 h; (b) ethyl 1,3-dioxoisoindoline-2-carboxylate, Et3N, CH2Cl2, rt, 15 h; (c) 3, Et3N, 1,4-dioxane–dimethyl sulfoxide (DMSO), 100 °C, 22 h, 52% over three steps; (vi) 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indazole, Pd(OAc)2, 1,1′-bis(di-tert-butylphosphino)-ferrocene, 2 M Na2CO3, 1,4-dioxane, 130 °C, 21 h, then NH2NH2·H2O, 100 °C, 48 h, 34%; (vii) amine, THF, 50 °C, 16 h, 29–85%; (viii) (a) tert-butyl ((1r,4r)-4-aminocyclohexyl)carbamate, Et3N, CH2Cl2, rt, 20 h; (b) HCl–1,4-dioxane, MeOH, rt, 2 h, 37% over two steps; (ix) 3, Et3N, 1,4-dioxane-DMSO, 50 °C, 23 h, 58%; (x) (a) isothiocyanatobenzene, EDC, THF, 50 °C, 5 h; (b) (Boc)2O, DMAP, THF, rt, 16 h; (c) bis(pinacolato)diboron, KOAc, Pd(dppf)Cl2·CH2Cl2, 1,4-dioxane, 135 °C, 24 h; (d) 3-bromo-6-chloro-N-((1-methylpiperidin-4-yl)methyl)imidazo[1,2-b]pyridazin-8-amine, Pd(OAc)2, 1,1′-bis(di-tert-butylphosphino)-ferrocene, 2 M Na2CO3, 1,4-dioxane, 135 °C, 16 h, 2–5% over four steps.