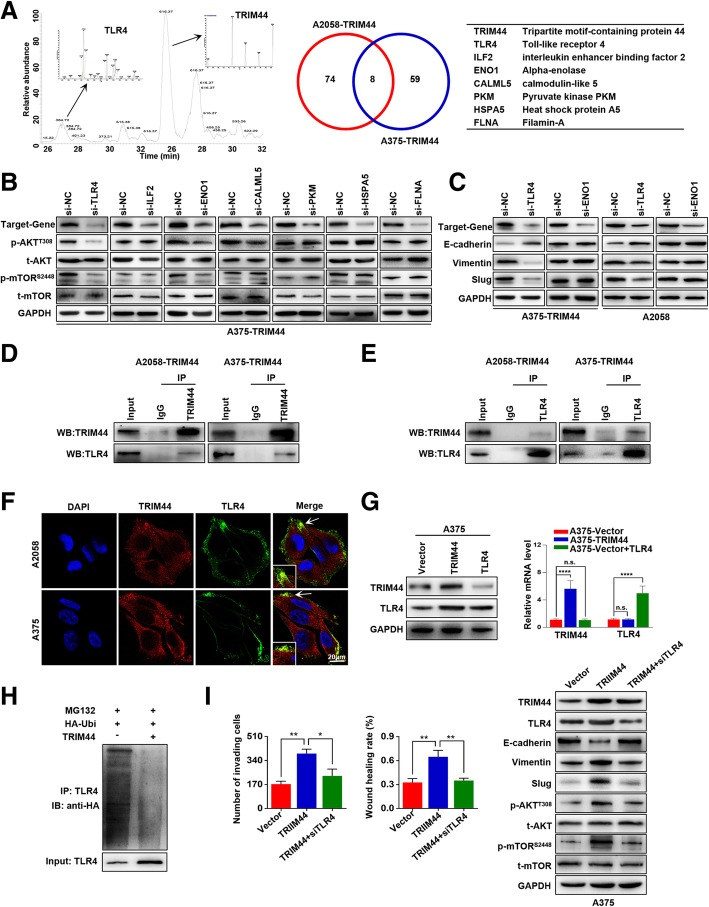
Fig. 5.
TRIM44 activates the AKT/mTOR pathway by blocking ubiquitination of TLR4. a, The binding partners of TRIM44 were analyzed by a combination of Co-IP and mass spectrometry, and seven overlapping proteins are presented. b, Western blot was used to quantify levels of p-AKT, AKT, p-mTOR and mTOR when the expression of relevant genes was inhibited. The AKT/mTOR pathway was affected by TLR4 and ENO1 interference. c, Western blot was used to detect levels of E-cadherin, Vimentin, and Slug in the indicated cells. Only TLR4 altered the expression of EMT markers. d and e, Co-IP used to verify formation of the TRIM44/TLR4 complex. f, Immunofluorescence used to verify formation of the TRIM44/TLR4 complex. g, Western blot and qRT-PCR used to quantify expression of TRIM44 and TLR4 in the indicated cells. TRIM44 overexpression led to upregulation of TLR4 proteins, but did not influence mRNA levels. h, Ubiquitination assay for the effects of TRIM44 on TLR4 ubiquitination. HA-Ubi were co-transfected into A375-Vector and A375-TRIM44 cells. i, Invasion and migration assays were performed in the indicated cells (left); Western blot was used to quantify expression levels of TRIM44, TLR4, EMT markers, p-AKT, AKT, p-mTORs2448 and mTOR in the indicated cells (right). After transfecting siTLR4 into A375-TRIM44 cells, TRIM44-induced EMT was significantly attenuated. n.s., not significant, *p < 0.05, **p < 0.01, ***p < 0.001