Figure 2.
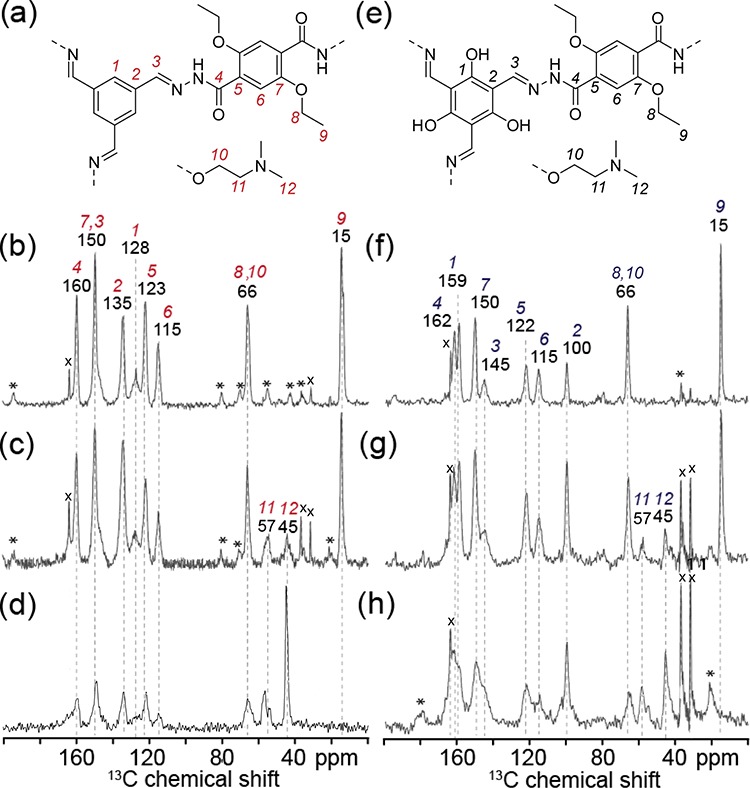
Schematic structural diagrams showing subsections of the (a) coCOF–H framework, (e) coCOF–OH framework, and the tertiary amine linker DtATH. Solid-state one-dimensional (1D) 13C{1H} CP-MAS NMR spectra of (b–d) coCOF–H and (f–h) coCOF–OH with (b,f) 0%, (c,g) 50%, and (d,h) 100% of DtATH substitution of the original DETH linker. The spectra in (b–d) and (f–h) were acquired at 11.7 T, 10 kHz MAS, 298 K, using cross-polarization contact times of 5 ms. The NMR spectrum (d) was acquired at 11.7 T, 12 kHz MAS, 298 K, and using cross-polarized contact times of 5 ms. Spinning sidebands are marked with asterisks. Distinct carbon atoms in the schematic structures in (a–e) are numbered and their associated 13C NMR signals labeled accordingly in (b–d) and (f–h). The narrow signals labeled with crosses at 164, 37, and 32 ppm correspond to residual dimethylformamide and at 25 ppm to residual tetrahydrofuran.46
