Figure 3. Histone modifications, miRs, and lncRNAs implicated in atherosclerosis.
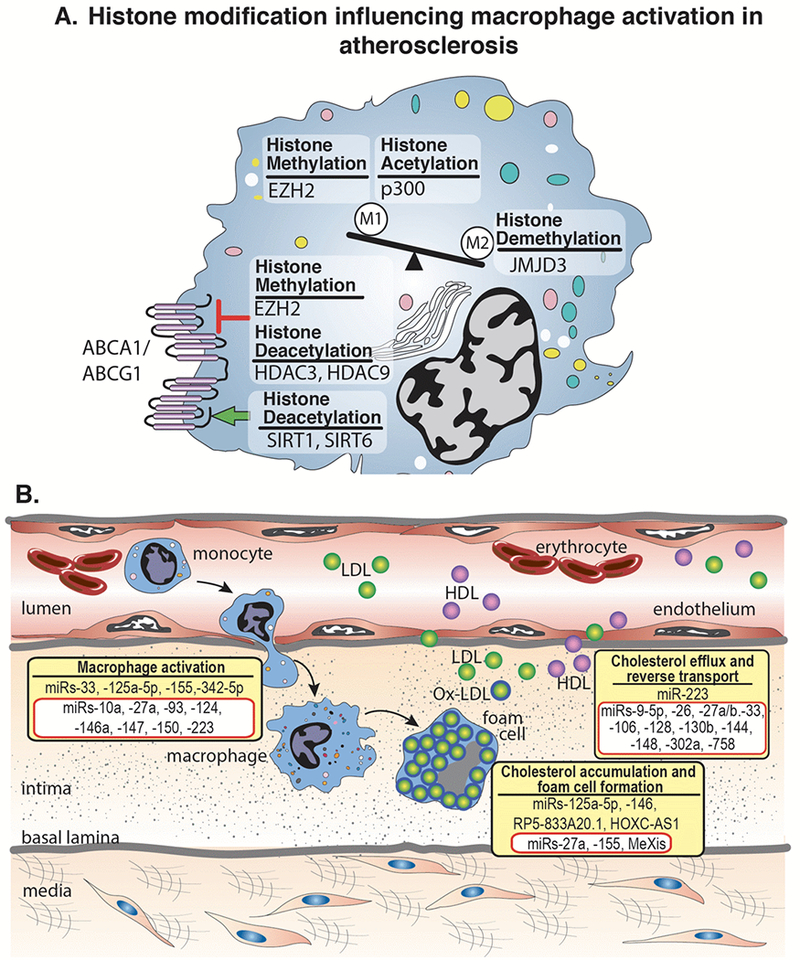
A. Histone modifying enzymes regulate the polarization of macrophages toward classical ‘M1’ or alternative ‘M2’ inflammatory activation. Within atherosclerosis specifically, studies have shown the histone methyl transferase, EZH2, and the histone acetytransferase, p300, polarize macrophages toward an ‘M1’ phenotype. In contrast, the histone demethylase, JMJD3, polarizes toward an ‘M2’ phenotype In addition, histone modifying enzymes can have stimulatory (green arrow) or inhibitory (red arrow) effects on ABCA1/ABCG1 altering cholesterol efflux and reverse cholesterol transport. B. The initial stages of atherosclerosis include adhesion of blood monocytes to the activated endothelium, their maturation into macrophages and their activation and uptake of ox-LDL to form macrophage-derived foam cells. Cholesterol efflux mechanisms, aided by ABCA1/ABCG1 and other factors, help to regress lesion development. miRs and lncRNAs exhibit diverse roles in atherosclerotic progression through the involvement of macrophage activation, foam cell formation, and cholesterol efflux. Representative miRs and lncRNAs that stimulate these processes are shown in yellow boxes. The miRs and lncRNAs highlighted in red boxes have been shown to inhibit macrophage activation, foam cell formation, or cholesterol efflux.
