Table 1.
Optimization of the key Suzuki-Miyaura coupling of vinyl bromide 5 and boronic monoester 4.a
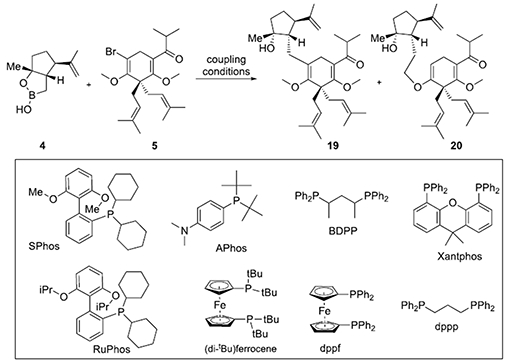 | ||||
|---|---|---|---|---|
| entry | Ligand (0.2 equiv) | Base (3 equiv) | Suzuki-Miyaura couplingb | C-H insertionb |
| 1 | SPhosc | Cs2CO3 | trace | 30% |
| 2 | SPhosd | Cs2CO3 | trace | 51% |
| 3 | PPh3 | Cs2CO3 | trace | 2% |
| 4 | Xantphos | Cs2CO3 | trace | 21% |
| 5 | dppp | Cs2CO3 | trace | trace |
| 6 | dppf | Cs2CO3 | trace | 17% |
| 7 | RuPhos | CsF | 47% | trace |
| 8 | SPhos | K2CO3 | 34% | 22% |
| 9 | SPhos | K3PO4 | 10% | 17% |
| 10 | APhos | Cs2CO3 | 40% | 12% |
| 11 | APhose,f | Cs2CO3 | 74% | trace |
Standard conditions: 10% Pd(OAc)2, ligand (0.2 equiv), base (3 equiv), PhMe (0.1M), 65 °C.
Yields refer to purified, isolated products.
Reaction temperature was 110 °C.
Concentration: 0.01M.
Slow addition of the catalyst (0.1 mL/h, over 3 h) as a toluene solution.
Concentration: 0.2 M.
