Fig. 6:|. Immunoglobulin class switch recombination.
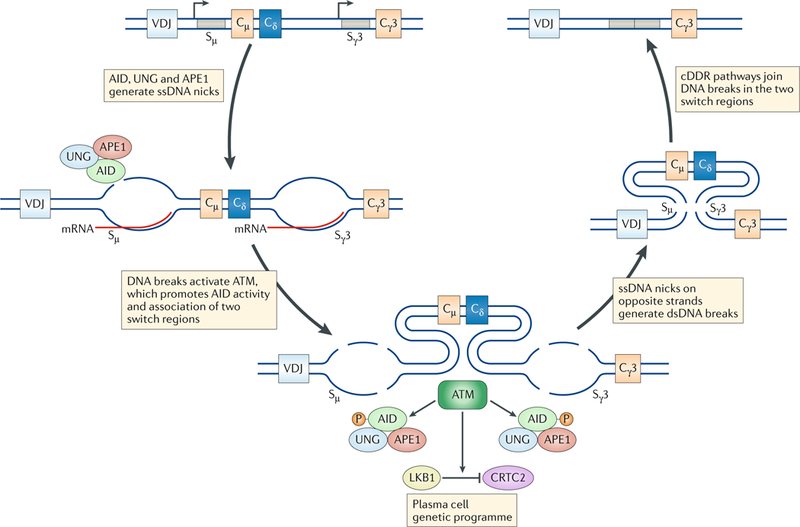
Immunoglobulin class switch recombination (CSR) is initiated by transcription through the switch μ (Sμ) region and a downstream switch region to which switching will occur (shown here to be Sγ3). Activation-induced deaminase (AID) targets the non-template single-strand DNA (ssDNA), deaminating cytosine to form uridine. The proteins uracil-DNA glycosylase (UNG) and apurinic/apyrimidinic endonuclease 1 (APE1) of the base excision repair pathway then deglycosylate and excise the uridine to generate a single-strand nick. The same reaction occurs on the template strand, although less efficiently, and the formation of closely staggered nicks on the two DNA strands leads to a DNA double-strand break (DSB). Broken DNA ends from the two switch regions are then joined by either classical or alternative non-homologous end joining. CSR DSBs activate ataxia telangiectasia mutated (ATM), initiating a non-canonical DNA damage response (ncDDR) that regulates CSR and B cell differentiation. Phosphorylation of AID by ATM in response to CSR DSBs promotes the association of APE1 with AID, leading to more efficient nick formation. ATM activity promotes the association of the two switch regions with DSBs. ATM activates the kinase LKB1, which inhibits CREB-regulated transcription co-activator 2 (CRTC2), a transcriptional co-activator of cAMP-responsive element-binding protein 1 (CREB1), promoting a plasma cell genetic programme. cDDR, canonical DNA damage response; dsDNA, double-strand DNA; P, phosphorylation.
