Abstract
The pro-inflammatory cytokine IL-1β is a secreted protein that is cleaved by caspase-1 during inflammasome activation upon recognition of internal and external insults to cells. Purinergic receptor P2X7 has been described to be involved in the release pathway of bioactive mature IL-1β by activated immune cells. Microparticle (MP) shedding has also been recently recognized as a manner of cytokine IL-1β release. However, the understanding of purinergic receptor roles in the MP-mediated IL-1β release process is still rudimentary. Gasdermin-D (GSDM-D), a protein involved in pyroptosis and inflammasome activation, has been recently described to be involved in the release of microparticles by virtue of its pore-forming ability. Hence, our current work is aimed to study the role of P2X7 in regulating GSDM-D-mediated microparticles and thereby bioactive IL-1β release. We provide evidence that cleaved functional IL-1β release in microparticles upon LPS stimulation is regulated by GSDM-D and P2X7 in a two-step fashion. GSDM-D activation first regulates release of IL-1β and P2X7 into microparticles. Then, microparticulate active P2X7 receptor then regulates the release of bioactive IL-1β encapsulated in microparticles to be able to target other cells inducing IL-8. Using an ATP model of stimulation, we further demonstrated that extracellular ATP stimulation to IL-1β containing LPS microparticles induces release of its content, which when subjected to epithelial cells induced IL-8. This effect was blocked by P2X7 inhibitor, KN62, as well as by IL-1RA. Taken together, our findings demonstrate for the first time the synergistic critical roles of GSDM-D and purinergic receptors in the regulation of microparticulate bioactive IL-1β release and induction of target cell responses.
Keywords: P2X7, GSDM-D, Microparticles, IL-1β, Monocytes, Inflammasome
The cytokine IL-1β is known to be the key initiator of acute inflammatory responses [1], which involves the activation of large protein complexes called inflammasomes. Inflammasomes mediate the conversion/maturation of pro-inflammatory interleukin (IL-1β and IL-18) proteins into mature/functionally active molecules involved in the promotion of inflammatory responses. The IL-1β-converting enzyme (ICE-1) or caspase-1 is an important regulator of this inflammatory response. Microparticles (MPs) are small membrane-coated structures that are released from the cells upon activation or during apoptosis [2, 3]. In addition to its classical role in IL-1β/IL-18 processing, caspase-1 has been demonstrated to play a role in microparticle-mediated cell death. Gasdermin-D (GSDM-D) has recently been shown to induce pyroptotic cell death. GSDM-D is a 487 amino acid cytoplasmic protein that contains an ill-characterized gasdermin domain and lacks any obvious transmembrane segment or signal peptide. GSDM-D has been shown to be cleaved from its inactive precursor p52 form by inflammatory caspases, and the cleaved active p30 amino terminal fragment GSDM-D is thought to be involved in the induction of pyroptotic cell death due to its pore-forming capacity. Our recent studies have demonstrated that monocyte-derived microparticles can induce cell death via active caspase-1 and p30 GSDM-D [4]. This cell death induction by the release of microparticles from stimulated cells is regulated by active p30 GSDM-D, shown to be involved in the release of the active caspase-1 encapsulated microparticles.
Microparticles have also been previously described as containing IL-1β, and microparticle shedding is recognized as a manner of cytokine release [5]. Work from other groups has shown that IL-1β-containing microparticles are a major secretory pathway from activated monocytes via early activation of P2X7 receptors [6, 7]. However, although the complex signaling and the role of the receptor are slowly beginning to be understood, there is a paucity of information regarding the function of P2X7 regarding microparticle formation and subsequent function. Here, we show that microparticles produced from early stimulation of monocytes/THP-1 cells following LPS challenge contain the P2X7 receptor, as well as components of the mature/functional inflammasome including active p20 caspase-1, active p30 GSDM-D, and mature IL-1β. Additionally, we show that the encapsulation of the P2X7 receptor in microparticles is essential for the release of encapsulated IL-1β to activate epithelial cells and induce the release of IL-8. In the absence of GSDM-D, this release of microparticulate IL-1β is completely abrogated. Hence, we propose that P2X7 regulates the release of active IL-1β encapsulated in microparticles in conjunction with active GSDM-D, an event essential for induction of cell activation and responses.
Materials and methods
Cells and reagents
Bacterial lipopolysaccharide E. coli strain (Serotype 0111:B4; Catalog # ALX-581-012, Lot # L27239) was obtained from Enzo Life Sciences (Farmingdale, NY). RPMI 1640 (Catalog # 10-040-CM) was purchased from MediaTech (Manassas, VA). Phosphate buffered saline (PBS, Catalog # 14190-144) was purchased from Gibco LifeSciences (Grand Island, NY), and fetal bovine serum (FBS, Catalog # F0500-D, Lot # 911042) was obtained from Atlas Biologicals (Fort Collins, CO). ATP was obtained from Sigma-Aldrich (Catalog # A6419; St. Louis, MO). Oxidized ATP was obtained from Sigma-Aldrich (Catalog # A6779; Lot # 120M5058V; St. Louis, MO). KN62 was obtained from Sigma-Aldrich (Catalog # I2142; St. Louis, MO). Human THP1 monocytic cells were obtained from American Type culture collection. THP1/Cas9 and THP1 Cas9/GSDM-D KO cells were generated by Dr. Seth Masters, WEHI, Australia [8]. Cells were routinely cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified CO2 incubator. Cells were cultured for 4 h in the presence or absence of LPS (1 μg/ml). BEAS2B cells were received from Dr. Knoell (The Ohio State University, Columbus, OH) and were plated at 100,000 cells/well in Costar 24-well Cell Culture Cluster (Corning, Inc.; Corning, NY). Co-culture experiments of BEAS2Bs with microparticles were performed in RPMI medium with 10% FBS throughout the experiments.
Microparticle isolation
Microparticle (MP) isolation was performed following our published protocol [9]. Briefly, culture media from cells stimulated or not with LPS (1 μg/ml) for 4 h were collected and centrifuged at 2000g for 5 min and 16,000g for 5 min to remove floating cells and cell debris. The culture media was further centrifuged at 100,000g for 1 h to isolate microparticles. Microparticles were then re-suspended in lysis buffer or RPMI based on the requirement of the experiment. Functional studies were then performed with the pelleted MPs by subjecting them to immunoblotting or co-culture assays with epithelial cells (BEAS2Bs).
Microparticulate quantification
MPs isolated from THP1 monocytic cells treated with LPS (LPS MP) or left untreated (control MP) were subjected to quantification analysis for normalization purposes. First, total proteins were measured from the MPs. MPs were then subjected to quantification using the NanoSight technology following the company manual. The Malvern NanoSight range of instruments utilizes nanoparticle tracking analysis (NTA) to characterize nanoparticles from 10 to 2000 nm in solution. Each particle is individually but simultaneously analyzed by direct observation and measurement of diffusion events. Based on our analysis, control and LPS MPs when normalized using protein quantification showed similar number of particles. Based on this observation, all MPs were analyzed based on protein normalization and added to epithelial cells for cell death assays.
Immunoblotting
Culture media was removed from cells and differentially centrifuged to collect cell pellets and microparticles. Cell pellets or microparticles were lysed in lysis buffer (50 mM Tris-Cl pH 8.0, 125 mM NaCl, 10 mM EDTA, 10 mM sodium fluoride, 10 mM sodium pyrophosphate, and 1% Triton X-100 containing protease inhibitor cocktail from Sigma and 50 μM N-methoxysuccinyl-Ala-Ala-Pro-Val chloromethylketone). The protein concentration in the cell lysates and microparticles was determined by Dc Lowry protein assay reagent (Bio-Rad). Equal amounts of total protein were resolved by SDS-PAGE and transferred to PVDF membrane. The membrane was then blocked with 10% nonfat dry milk in TBST (25 mM Tris-HCl pH 7.5, 150 mM NaCl, .1% Tween 20) for 2 h at room temperature. The membranes were then probed with primary antibodies as indicated followed by peroxidase-conjugated secondary antibodies. Protein bands were visualized by enhanced chemiluminescence (ECL, GE Healthcare). Densitometry scans were performed using Image J (NIH) from immunoblots of n = 3 experiments. Analysis was performed by using ratios of specific proteins to loading control Hsp90 for each immunoblot.
Elisa
Sandwich ELISAs were developed in our laboratory to detect mature IL-1β. Briefly, anti-human mouse monoclonal antibody (clone 2805, R&D Systems) was used as a coating antibody and a rabbit polyclonal mature IL-1B (raised against entire 17-KDa mature IL-1β) was used, respectively. Horseradish peroxidase (Bio-Rad)–conjugated goat anti-rabbit antibody was used as a developing antibody. IL-8 was examined by the use of eBioscience IL-8 ELISA ready-SET-Go! (2nd Generation) (Catalog # 88-8086-88) following the manufacturer’s protocol.
Statistical analysis
Data are represented as the mean + standard error of the mean (SEM) from at least three independent experiments. p < 0.05 was considered to represent statistical significance.
Results
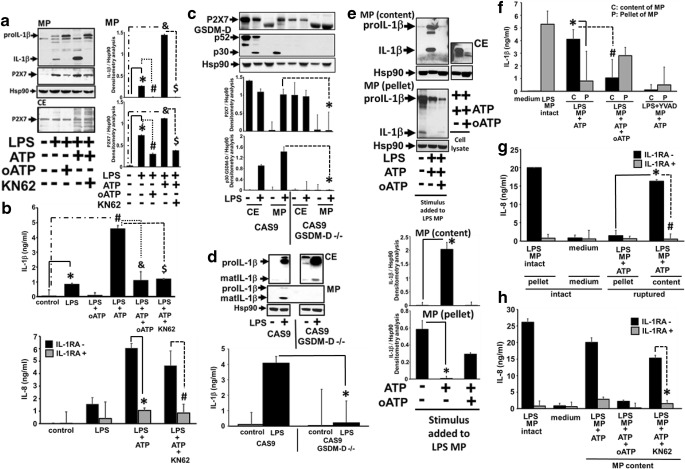
P2X7 is known to be responsible for the ATP-mediated IL-1β release from activated monocytes. This role of P2X7 in regulating IL-1β release has also been confirmed using transgenic P2X7-lacking mice where IL-1β release was abrogated from monocytes primed with LPS [10]. GSDM-D recently has been shown to regulate IL-1β release as well as active caspase-1 release in microparticles. GSDM-D KO mice are deficient in release of IL-1β [11, 12]. Our own previous work has demonstrated that active GSDM-D is packaged in microparticles along with active caspase-1 upon stimulation of THP1 monocytic cells with LPS. We have also demonstrated that the absence of GSDM-D abrogates the release of microparticulate active caspase-1, although the activation process remains unhindered [4]. Hence, our current work is aimed to understand the role of GSDM-D and P2X7 in regulation of microparticulate IL-1 β and its ability to induce lung epithelial cells. As seen in Fig. 1a, P2X7 was detected along with mature IL-1β in microparticles released from THP1 stimulated with LPS (1 μg/ml) for 4 h in the presence or absence of ATP. This release of P2X7 and IL-1β was significantly higher in LPS or LPS/ATP-induced MPs (p < 0.05). Although this MP release of mature IL-1β was completely abrogated by P2X7 inhibitor, KN62 or oxidized ATP (p < 0.05), release of proIL-1β was still detected in the MPs. The released microparticulate IL-1β, when subjected to epithelial cells, resulted in increased IL-8 production from the epithelial cells (p < 0.05), which was completely abrogated in the presence of IL-1RA suggesting that the microparticulate IL-1β is functional (p < 0.05) (Fig. 1b). Since GSDM-D was also detected in MPs containing mature IL-1β, and GSDM-D knockout cells have been demonstrated to lack IL-1β release, we performed experiments to test the role of GSDM-D in the P2X7-mediated microparticulate IL-1β release. We used CRISPR/Cas9 GSDM−/− cells and analyzed for the presence of P2X7 and IL-1β in both MP and cytosolic fractions. Surprisingly, we observed that microparticulate P2X7 release was significantly abrogated in GSDM-D−/− cells as compared to CAS9 cells (p < 0.05), although P2X7 was detected in the cytosolic fractions (Fig. 1c), suggesting that GSDM-D regulated the release of microparticles containing P2X7. As expected, mature IL-1β release in microparticles was also abrogated in GSDM−/− cells (Fig. 1d), despite of the presence of functional cytosolic P2X7 (Fig. 1c) and no inhibition of IL-1β processing in GSDM−/− cell lysates (CE) (Fig. 1d) upon LPS stimulation. We then analyzed the role of microparticulate P2X7 in the release of bioactive IL-1β to target cells. LPS MPs containing mature IL-1β were treated with exogenous ATP (5 mM) or were left untreated, and the contents of ruptured MPs were analyzed. As seen in Fig. 1e, f, ATP stimulation of LPS MPs completely released mature IL-1β into the soluble fractions by rupturing the LPS MPs (p < 0.05). Intact MPs detected IL-1β in the MP pellet fraction. This release of mature IL-1β into the content fraction by exogenous ATP was significantly abrogated by oATP and YVAD (specific inhibitor of caspase-1) (Fig. 1e, f) (p < 0.05). Both intact MPs and ruptured content of MPs (containing bioactive IL-1β) were then used to treat epithelial cells, which were then analyzed for bioactive IL-1β-mediated IL-8 inductions. As seen in Fig. 1g, contents of ATP-ruptured LPS MPs containing mature IL-1β significantly induced IL-8 responses (p < 0.05), comparable to intact LPS MPs, which was significantly inhibited by IL-1RA (p < 0.05). This effect was also abrogated by blocking microparticulate P2X7 using KN62 (Fig. 1h).
Fig. 1.
Microparticulate P2X7 regulates IL-1β release and responses to target cells via active p30 GSDM-D. a THP1 cells were stimulated LPS (1 μg/ml) for 4 h in the presence or absence of ATP, oATP, and KN62. MPs were isolated from supernatants and analyzed for the presence of mature IL-1β and P2X7. b Densitometry scans of mature IL-1β and P2X7 are from n = 3 experiments. *LPS MP compared to control, #LPS + oATP vs LPS, and LPS + ATP vs control and $ LPS + ATP + KN62 vs LPS + ATP B) Top panel: Mature IL-1β was measured from supernatants from experimental conditions described in (a). *LPS MP compared to control, # LPS + ATP vs control, and LPS + ATP + oATP vs LPS + ATP and $ LPS + ATP + KN62 vs LPS + ATP. Bottom panel: Same fractions were also subjected to healthy BEAS2B cells and measured for IL8 release in the presence or absence of IL-1RA.*LPS + ATP + IL-RA compared to LPS + ATP-IL-RA, # LPS + ATP + KN62 + IL-1RA vs LPS + ATP + KN62-IL-1RA. c, d CAS9 and CAS9/GSDM-D−/− cells were stimulated with LPS for 4 h. Cell lysates (CE) and microparticles (MPs) were measured for the presence of P2X7 and mature IL-1β. Densitometry scans of P2X7 and mature IL-1β from n = 3 experiments were performed. Supernatants were also analyzed for mature IL-1β by ELISA. *GSDM-D −/− LPS MP vs CAS9 LPS MP. e, f LPS MPs were generated from THP1 cells stimulated with LPS (1 μg/ml) for 4 h. LPS MPs were then either left intact or treated with ATP or oATP for 30 min and then spun for 100,000g for 11/2 h to separate broken MP pellet and MP content. Pellet and content fractions were then probed for the presence of pro and mature IL-1β using immunoblot and ELISA. *LPS + ATP content vs LPS + ATP pellet IL-1β levels, #LPS + ATP vs LPS + ATP + oATP content IL-1β levels. g Intact LPS MP, MP pellet, and MP mature IL-1β contents by rupturing intact MPs using ATP were subjected to BEAS2B cells for 24 h, and release of IL-8 was measured. *LPS MP + ATP content vs LPS MP + ATP pellet induced IL-8 levels, #LPS MP + ATP content − IL-1RA vs LPS MP + ATP content + IL-1RA. h IL-8 release from BEAS2B cells by intact LPS MP, contents of LPS MP + ATP, LPS MP treated with ATP + oATP, and LPS MP treated with ATP and KN62 were measured in the presence or absence of IL-1RA. *LPS MP + ATP + KN62 content − IL-1RA vs LPS MP + ATP + KN62 content + IL-1RA
Discussion
Microparticle-related inflammasome release may represent a novel form of cell to cell communication in response to pathogen challenge [13]. It is becoming increasingly clear that microparticle release of IL-1β and inflammasome proteins are tightly regulated. In the current work, we evaluate the role of purinergic receptor, P2X7, and GSDM-D in the regulation of microparticulate IL-1β release. We provide clear evidence that GSDM-D and P2X7 regulate the release of microparticulate IL-1β release from cells and its ability to target cells in a two-step process. Using GSDM-D−/− cells, our novel work clearly demonstrates that cytosolic GSDM-D regulates the release of IL-1β and P2X7 from cells upon LPS stimulation. As a result, no release of microparticulate IL-1β was observed from GSDM-D KO cells when compared to CAS9 cells. P2X7 and active GSDM-D were packaged in MPs upon stimulation of THP1 cells with LPS along with mature IL-1β and active caspase-1. This data support the hypothesis that the P2X7 receptor regulates the release of MP-encapsulated IL-1β to target cells. Further supporting this hypothesis, the release of IL-1β from LPS MPs was blocked by KN62 or by oATP. Taken together, as seen in Fig. 2, our current work demonstrates that IL-1β release from stimulated THP1 cells is regulated by GSDM-D, and P2X7 is a dual-step process. GSDM-D activation first regulates the release of IL-1β and P2X7 into microparticles from stimulated cells and then microparticulate active P2X7 receptor regulates bioactive mature IL-1β release from the microparticles to target cells.
Fig. 2.
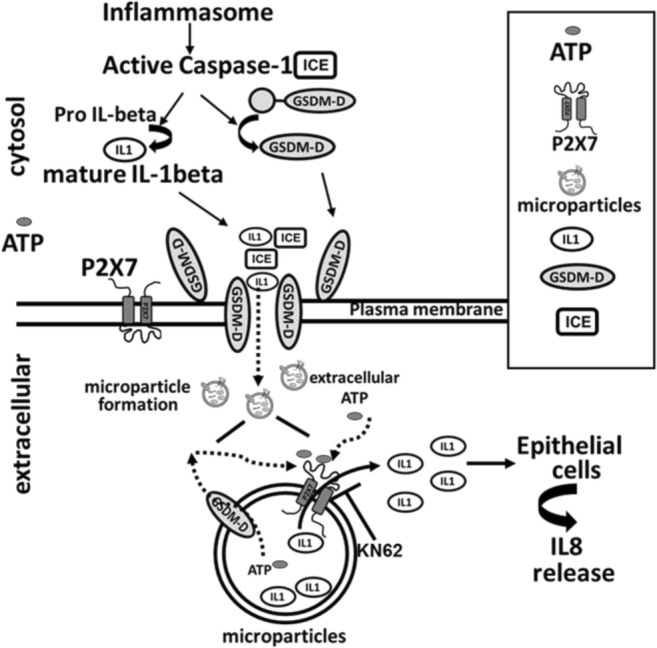
Proposed model for P2X7 and GSDM-D-mediated microparticulate IL-1β release. Microparticulate IL-1β release regulated by GSDM-D and P2X7 is a two-step process. GSDM-D activation regulating the release of IL-1β and P2X7 into microparticles, followed by microparticulate active P2X7 receptor regulating bioactive mature IL-1β release from the microparticles to target cells
The mechanism by which P2X7 and GSDM-D regulate MP-encapsulated IL-1β release is just beginning to be understood. It has been previously demonstrated that GSDM-D regulates cell death by forming pores that compromise the integrity of the cell membrane and release of calcium, as well as cytokine release [14–16]. Our group has recently described the novel role of caspase-1 in regulating GSDM-D activation and thereby regulating the MP caspase-1–mediated cell injury [4]. Much remains to be understood about the upstream signaling regulating sensing, activation, and MP release. It will be important to determine the factors regulating P2X7 activation. What happens to microparticulate P2X7 upon stimulation? Is GSDM-D playing an important role in membrane reconstitution and formation of microparticles directly and thereby regulating IL-1β and P2X7 release? It is clear that our current novel findings support the need for further studies understanding the role of P2X7 in microparticle-mediated IL-1β release and responses to target cells.
Funding information
This work was supported by NIH RO1 HL24325 to Sarkar and NIH RO1GM108928 to Sarkar/Wewers.
Conflicts of interest
Srabani Mitra declares that she has no conflict of interest.
Anasuya Sarkar declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8(4):253–265. doi: 10.1016/S1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 2.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticle: two sides of the coin. Physiology. 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 3.Distler JH, Huber LC, Gay S, Distler O, Pesetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39(8):683–690. doi: 10.1080/08916930601061538. [DOI] [PubMed] [Google Scholar]
- 4.Mitra S, Exline M, Habyarimana F, Gavrilin M, Baker P, Masters SL, Wewers MD, Sarkar A. Microparticulate caspase-1 regulates gasdermin-D and pulmonary vascular endothelial cell injury. Am J Respir Cell Mol Biol. 2018;59:56–64. doi: 10.1165/rcmb.2017-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179(3):1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 6.MacKenzie A, Wilson HL, Kriss-Toth A, Dower SK, North A, Surprenant A. Rapid secretion of interleukin-1 beta by microvesicle shedding. Immunity. 2001;8:825–835. doi: 10.1016/S1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 7.Wewers MD, Sarkar A. P2X7 receptor and macrophage function. Purinergic Signal. 2009;5:189–195. doi: 10.1007/s11302-009-9131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin HY, Lin PH, Wu SH, Yang LT. Inducible expression of gasdermin A3 in the epidermis causes epidermal hyperplasia and skin inflammation. Exp Dermatol. 2015;24(11):897–899. doi: 10.1111/exd.12797. [DOI] [PubMed] [Google Scholar]
- 9.Mitra S, Wewers MD, Sarkar A. Mononuclear phagocyte-derived microparticulate caspase-1 induces pulmonary vascular endothelial cell injury. PLoS One. 2015;10(12):e0145607. doi: 10.1371/journal.pone.0145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276(1):125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 11.Feng S, Fox D, Ming Man S. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol. 2018;430(18 Pt B):3068–3080. doi: 10.1016/j.jmb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Schneider KS, Groß CJ, Dreier RF, Saller BS, Mishra R, Gorka O, Heilig R, Meunier E, Dick MS, Ćiković T, Sodenkamp J, Médard G, Naumann R, Ruland J, Kuster B, Broz P, Groß O. The inflammasome drives GSDMD-independent secondary pyroptosis and IL-1 release in the absence of caspase-1 protease activity. Cell Rep. 2017;21(13):3846–3859. doi: 10.1016/j.celrep.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009;4(9):e7140. doi: 10.1371/journal.pone.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. Gasdermin (GSDM-D) localizing to mouse chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm Genome. 2007;11(9):718–724. doi: 10.1007/s003350010138. [DOI] [PubMed] [Google Scholar]
- 15.Russo HM, Rathkey J, Boyd-Tressler A, Katsnelson MA, Abbott DW, Dubyak GR. Active caspase-1 induces plasma membrane pores that precede pyroptotic lysis and are blocked by lanthanides. J Immunol. 2016;197(4):1353–1367. doi: 10.4049/jimmunol.1600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKechnie NM, King BCR, Fletcher E, Braun G. Fas-ligand is stored in secretory lysosomes of ocular barrier epithelia and released with microvesicles. Exp Eye Res. 2006;83:304–314. doi: 10.1016/j.exer.2005.11.028. [DOI] [PubMed] [Google Scholar]