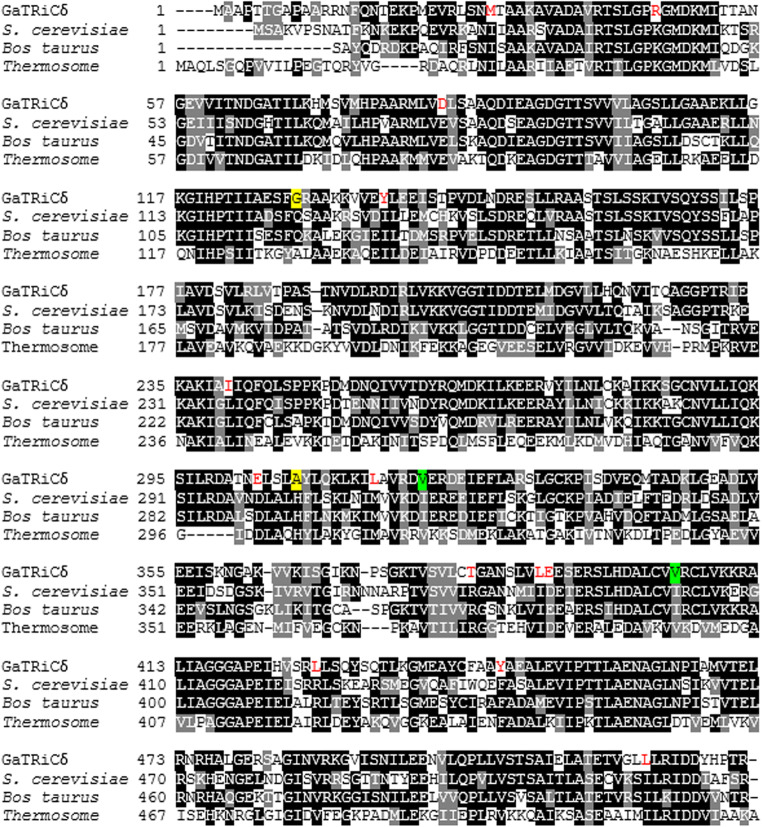
Fig. 4.
Sequence alignment between TRiCδ from G. antarctica, S. cerevisiae, B. taurus, and Thermococcus. The amino acid sequences of the TRiC δ-subunit from G. antarctica was realigned relative to those of S. cerevisiae (PDB: 4V81_D), B. taurus (PDB: 4B2T_D) and Thermococcus (PDB: IA6D_D). Highly conserved residues are highlighted in black while partly conserved in gray. Residue substitutions of the GaTRiCδ that differ with respect to the reference sequences are shown in red and significant changes in the tertiary structure interactions are highlighted in yellow for ionic interaction while green for hydrophobic interaction