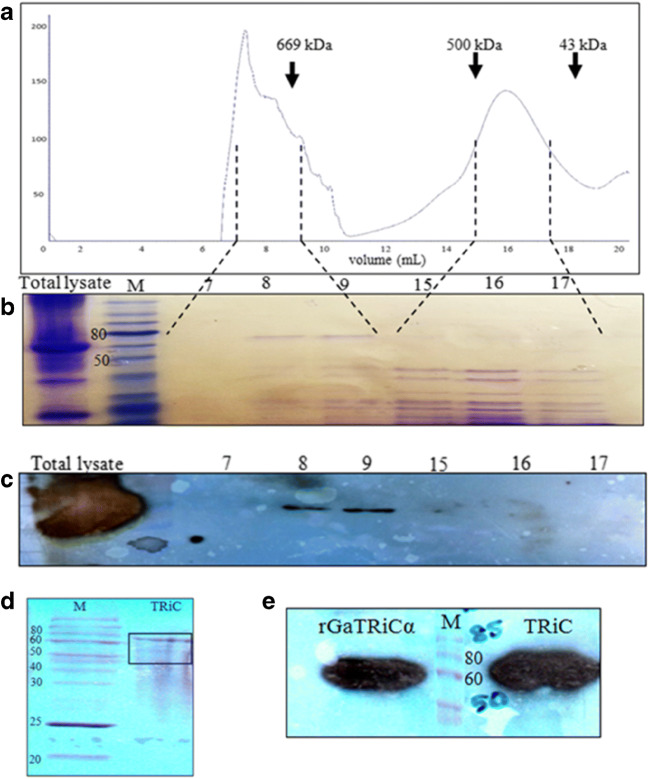
Fig. 7.
Size exclusion chromatography of G. antarctica TRiC. a A chromatogram of gel purification using Sepharose 6 with the arrowheads showing the positions of molecular size markers (thyroglobulin, 669 kDa; ferritin, 440 kDa and ovalbumin, 43 kDa). The numbers 0 mL to 20 mL in the chromatogram represented the eluted fractions. b A SDS-PAGE analysis of the fractions collected from the gel filtration column. The lane numbered as 7, 8, 9, 15, 16, and 17 were the chosen eluted fractions that correspond to the size of TRiC complex. c An immunoblot analysis of fractions from the Superose 6 column using anti-TRiCα antibodies. The lane numbered as 7, 8, 9, 15, 16, and 17 corresponding to the chosen eluted fractions. d Protein composition of fractions 7–9 from the Superose 6 column pooled and concentrated by ultrafiltration to 1 mg/mL. The bands in the gel were revealed by Coomassie blue staining. e Immunoblot analysis of purified G. antarctica TRiC complex using anti-TRiCα antibodies. Recombinant G. antarctica TRiCα was running together as a positive control