Fig. 1.
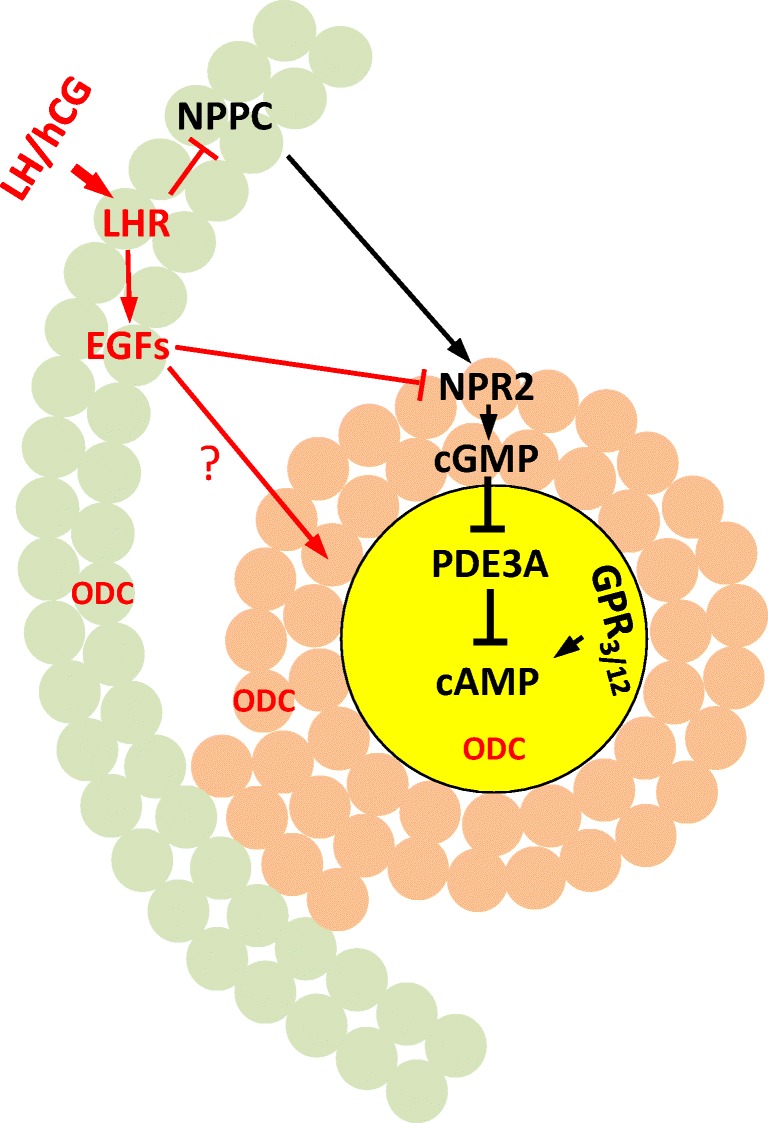
Mammalian oocyte meiosis arrest and oocyte maturation. A section of antral follicle including oocyte (yellow), cumulus cells (brown), and mural granulosa cells (green). Prophase maintenance signaling pathways are depicted in black and LH/hCG-induced signaling in red. Oocyte cAMP is produced by constitutively active Gs-coupled receptors GPR3 and GPR12. Cyclic AMP-specific phosphodiesterase, PDE3A, is kept inactive by cGMP, produced by a paracrine signaling involving mural granulosa cell-derived natriuretic peptide precursor type C (NPPC) and its receptor NPR2 (which is a guanylyl cyclase) and transported to the enclosed oocyte through gap junctions. Luteinizing hormone (LH) activates LH receptor in mural granulosa cells leading to the expression of several EGF-like peptides, which in turn cause rapid inactivation of NPR2 ending cGMP production. The lack of cGMP releases PDE3A to hydrolyze cAMP in the oocytes. LH surge also inhibits NPPC expression, thus shutting down cGMP production permanently. LH surge also triggers a brief (lasting several hours in rats and mice) and robust expression of ornithine decarboxylase (ODC) in all three components of the antral follicles [3, 58], producing high levels of putrescine in the ovaries
