Figure 3.
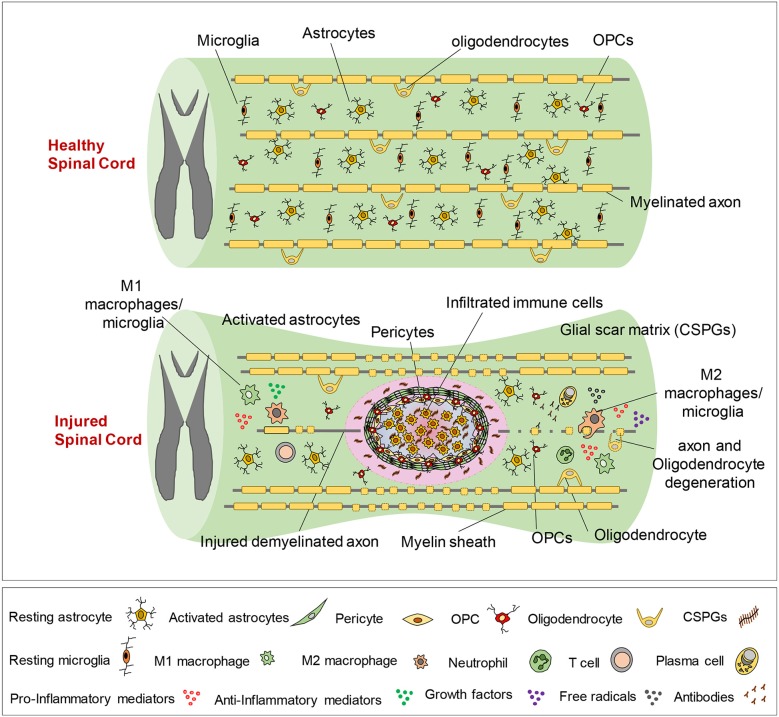
Pathophysiology of traumatic spinal cord injury. This schematic diagram illustrates the composition of normal and injured spinal cord. Of note, while these events are shown in one figure, some of the pathophysiological events may not temporally overlap and can occur at various phases of SCI, which are described here. Immediately after primary injury, activation of resident astrocytes and microglia and subsequent infiltration of blood-borne immune cells results in a robust neuroinflammatory response. This acute neuroinflammatory response plays a key role in orchestrating the secondary injury mechanisms in the sub-acute and chronic phases that lead to cell death and tissue degeneration, as well as formation of the glial scar, axonal degeneration and demyelination. During the acute phase, monocyte-derived macrophages occupy the epicenter of the injury to scavenge tissue debris. T and B lymphocytes also infiltrate the spinal cord during sub-acute phase and produce pro-inflammatory cytokines, chemokines, autoantibodies reactive oxygen and nitrogen species that contribute to tissue degeneration. On the other hand, M2-like macrophages and regulatory T and B cells produce growth factors and pro-regenerative cytokines such as IL-10 that foster tissue repair and wound healing. Loss of oligodendrocytes in acute and sub-acute stages of SCI leads to axonal demyelination followed by spontaneous remyelination in sub-acute and chronic phases. During the acute and sub-acute phases of SCI; astrocytes, OPCs and pericytes, which normally reside in the spinal cord parenchyma, proliferate and migrate to the site of injury and contribute to the formation of the glial scar. The glial scar and its associated matrix surround the injury epicenter and create a cellular and biochemical zone with both beneficial and detrimental roles in the repair process. Acutely, the astrocytic glial scar limits the spread of neuroinflammation from the lesion site to the healthy tissue. However, establishment of a mature longstanding glial scar and upregulation of matrix chondroitin sulfate proteoglycans (CSPGs) are shown to inhibit axonal regeneration/sprouting and cell differentiation in subacute and chronic phases.