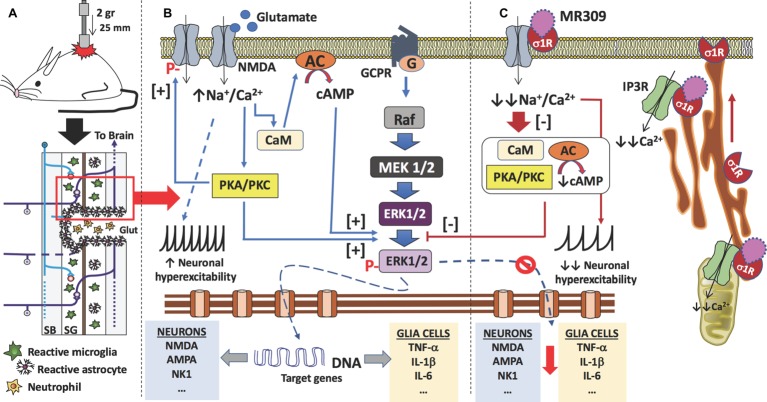
Figure 5.
Proposed mechanism of action of MR309 to decrease the expression of inflammatory cytokines and the hyperexcitability of spinal neurons after contusion of the spinal cord. (A) Contusion of the spinal cord of the mouse causes a spinal cord injury which results in glutamate (Glut) release by necrotic death of neurons and glial cells, medullary parenchyma destructuring, progressive reactivation of microglia and astrocytes, and the formation of the glial scar that surrounds the area of injury. In this injured area, there is also an infiltration of blood-forming elements, such as neutrophils, lymphocytes, and monocytes, is evidenced. In the neurons and glial cells surviving in the spinal cord injury (red box), the excess of glutamate in the medullary parenchyma causes a set of molecular changes. (B) The glutamate can interact with the NMDA receptors present in neurons and glial cells, causing an increase in the influx of sodium and calcium ions. In both cell types, the increase in intracellular calcium ions causes the activation of calmodulin (CaM), so that the calcium-calmodulin complex in turn activates adenylate-cyclase (AC) with an increase in the synthesis of cAMP, the second messenger that causes the activation (phosphorylation) of ERK1/2. Also, calcium ions activate protein kinases (PKA and PKC), which in turn also facilitate the phosphorylation of ERK1/2. Phosphorylated ERK1/2 translocates to the nucleus and transcription of target genes begins. In the case of spinal nociceptive neurons, genes encoding neurotransmitter receptors (e.g., AMPA, NMDA, and NK1) as well as voltage-gated ion channels are transcribed. While in the case of glial cells (astrocytes and microglia), genes encoding pro-inflammatory cytokines are transcribed (e.g., TNF-α, IL-1β, and IL-6). On the other hand, the influx of sodium ions causes an increase in the neuronal excitability of the spinal nociceptive neurons, with an increase in action potentials by the spinothalamic tract toward the brain. Gene transcription of ion channels and receptors for neurotransmitters also facilitates neuronal hyperexcitability. Finally, activated protein kinases also cause phosphorylation of NMDA-receptors that will further contribute to central sensitization. (C) σ₁ receptor classified as a ligand-regulated molecular chaperone is activated under stress or pathological conditions and interacts with several neurotransmitter receptors and ion channels to modulate their function and its activity can be regulated by endogenous and/or synthetic compounds in an agonist-antagonist manner (Díaz et al., 2009). Upon σ₁R activation under stress or pathological conditions (SCI in this case), σ1R in the ER binds to IP3R to enhance Ca2+ influx into mitochondria and efflux into the cytosol. There is also redistribution of σ1R from mitochondria-associated endoplasmic reticulum membrane to peripheral endoplasmic membranes to bind ion channels, receptors (NMDA) or protein kinases which, in turn will produce a further increase in intracellular Ca2+. Therefore, σ1R antagonism by MR309 would result in a reduced Ca2+ cytosolic mobilization from ER stores (via PLC and IP3R) and in a reduced extracellular entry through NMDAR, favoring the access of negative regulators of the receptor such as Ca2+-CaM, finally resulting in an inhibition of Ca2+ dependent intracellular effectors such as PKC/PKA and CaMKII. This downstream modulation would modulate the ERK pathway by preventing the phosphorylation of ERK1/2, and consequently, the levels of gene expression of NMDAR and proinflammatory cytokines, together with a decrease in the overactivity of NMDAR (mediated by phosphorylations at S1303 and Y1472). All of these changes would decrease neuronal and glial hyperexcitability and thus produce an attenuation in central neuropathic pain development.