Figure 1.
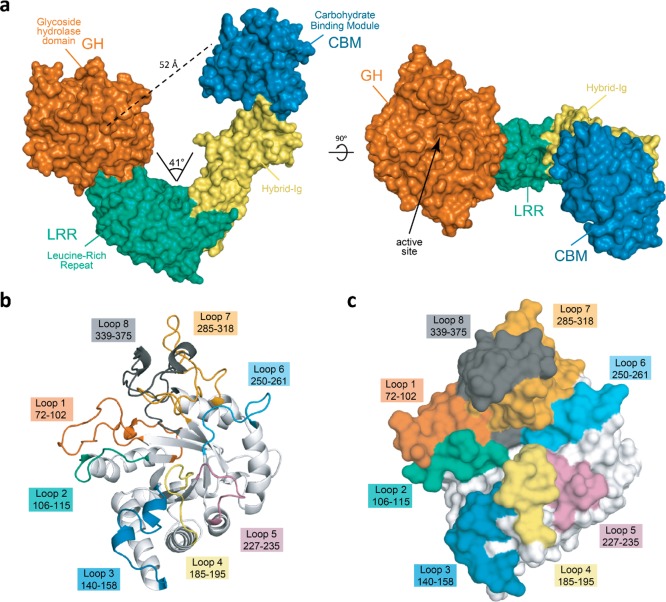
Overall architecture of EndoS2. (a) Overall structure of EndoS2 with annotated domains. The annotated distance is measured from the Cα of a conserved catalytic glutamate in the GH domain (E186) to the Cα of a conserved tryptophan in the CBM (EndoS2 W712). The annotated angle is between these two residues and the Cα of the first residue in the hybrid-Ig domain, near the base of the hinge (E548). (b) Cartoon and (c) surface representations showing the overall topology and annotation of GH domain loops.