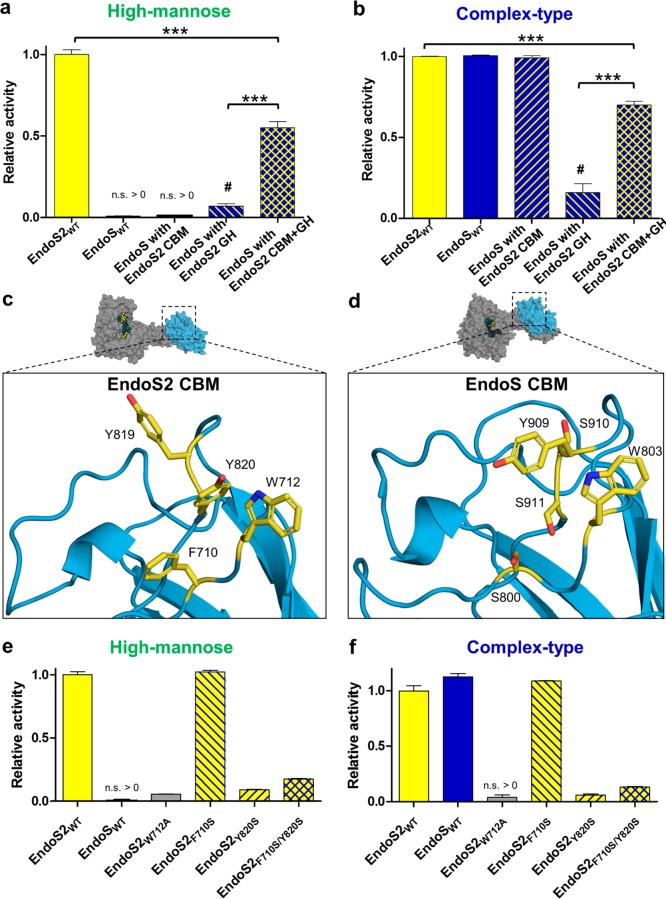
Figure 5.
Hydrolytic activity of chimeric domain-swapped enzymes. (a) Hydrolytic activity of enzymes toward IgG1 bearing high-mannose N-glycans and (b) complex-type N-glycans. Activity was measured using mass spectrometry, normalized to wild-type EndoS2. Reactions were performed in technical duplicate, and error bars represent standard deviation. Statistical significance compared to wild-type EndoS2 is annotated (multiple comparisons test, Tukey method; ***, p < 0.001; #, p < 0.05 compared to no-enzyme control; n.s. > 0, not significantly greater than no-enzyme control). Comparison of glycan-binding surfaces from (c) EndoS2 and (d) EndoS (PDB 4NUZ).19 The relative activity of specific point mutants intended to make EndoS2 more EndoS-like was tested against (e) high-mannose and (f) complex-type IgG1.