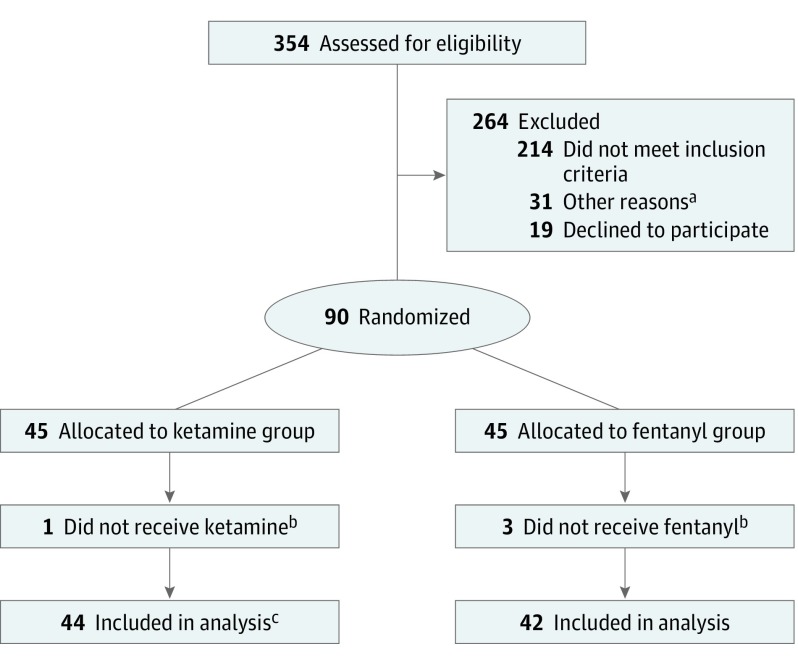
Figure 1. CONSORT Flow Diagram.
aOther reasons: research coordinator was not present, patient was not in proper location, study enrollment was on hold for regulatory purposes, and clinician preference.
bReasons for withdrawal: inability to provide urine for pregnancy test, parental preference changed, clinician preference, and unavailability of medication.
cFor 1 patient, only secondary outcome data were reported owing to missing baseline visual analog scale score. Therefore, 43 had primary outcome measures available.