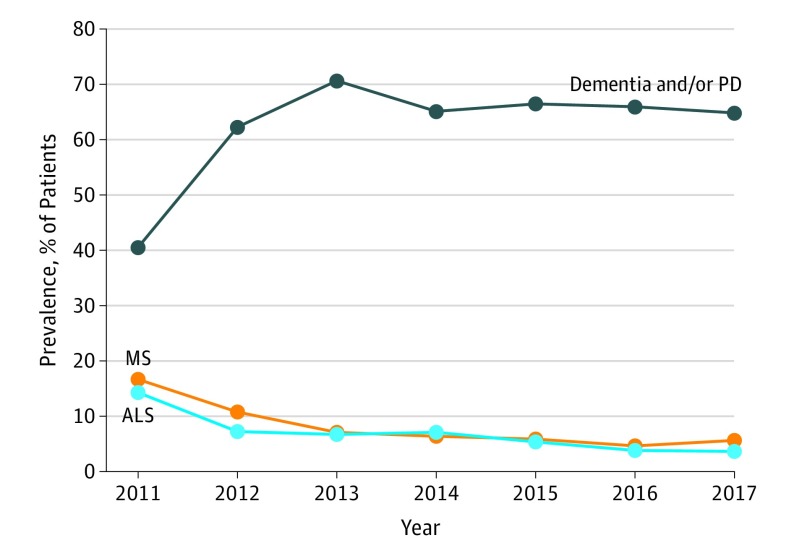
Figure 1. Changes Over Time in Main Associated Diagnoses Among Study Patients in the Optum Database.
Includes patients with amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), or dementia and/or Parkinson disease (PD) who received a prescription for dextromethorphan hydrobromide and quinidine sulfate from 2011 through 2017. The diagnoses were identified before and within 30 days after a prescription claim for the combination drug dextromethorphan-quinidine in the Optum Clinformatics Data Mart database. The exact periods represented by each year were October 29, 2010, through September 30, 2011 (2011); October 1, 2011, through September 30, 2012 (2012); October 1, 2012, through September 30, 2013 (2013); October 1, 2013, through September 30, 2014 (2014); October 1, 2014, through September 30, 2015 (2015); October 1, 2015, through September 30, 2016 (2016); and October 1, 2016, through March 1, 2017 (2017).