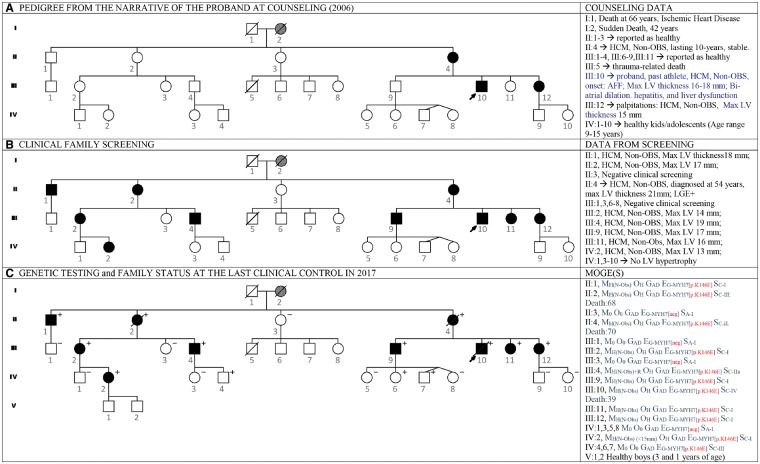
Figure 1.
The figure shows the pedigree of a family with autosomal dominant hypertrophic cardiomyopathy, the diagnostic impact of clinical family screening, and the phenotype–genotype description. The proband, III: 10, was diagnosed with hypertrophic cardiomyopathy at the age of 30 years, when he developed heart failure with atrial fibrillo-flutter (AFF). His medical history was characterized by recurrence of the atrial arrhythmias, ablations, and further recurrence of atrial fibrillo-flutter. He further suffered pneumonia with sepsis, pleuritis, and pericardial effusion, liver failure and progressed to end-stage heart failure. (A) The panel shows the first pedigree of the family, before performing clinical family screening and genetic testing. According to the narrative of the proband and to the health status of his relatives the only affected family members were II: 4, and III: 12. His grand-mother was probably affected (I: 2). (B) The panel shows the pedigree after completion of family screening: seven additional members were affected and asymptomatic, all unaware of their disease status. (C) The panel shows the pedigree after the completion of genetic testing: there are three healthy carriers in the third generation. Individual IV: 2 decided to have pregnancy and was supported and monitored during both pregnancy and delivery; her children are healthy and have not been tested yet. In the right column, phenotype and cause of the cardiomyopathies are precisely described using MOGE(S) nosology.14 In the right column, the summary of clinical data shows that genetically negative family members are not affected, supporting the segregation of the genotype with the phenotype up to the third generation. Future clinical monitoring of the family will demonstrate whether the healthy carriers of the fourth generation will develop the disease, further confirming the segregation of the phenotype with the genotype in the family. This is especially important in this family because the mutation identified in MYH7 is not reported.