Abstract
Cardiac resynchronization therapy (CRT) is an established treatment for patients with heart failure (HF), impaired left ventricular (LV) function, and wide QRS complex. The initial randomized clinical trials, which led to the widespread use of CRT, selected patients on the basis of QRS duration, not focusing on QRS morphology. However, recent evidences emphasized the role of left bundle branch block morphology in patients that underwent CRT in order to predict better response to therapy. Moreover, conventional right ventricular apical pacing might have long-term detrimental effects on cardiac structure and LV function, possibly leading to the development of HF. Therefore, current guidelines recommend upgrade from conventional pacemaker or implantable cardioverter-defibrillator to CRT or de novo CRT in patients with high (or expected high) percentage of ventricular pacing and reduced EF. We reviewed current knowledge on candidates’ selection for CRT based on conduction delays that lead to electrical and mechanical dyssynchrony of the left ventricle.
Keywords: Cardiac resynchronization therapy, Left bundle branch block, Right ventricular pacing
Introduction
Cardiac resynchronization therapy (CRT) is an established treatment for patients with heart failure (HF), impaired left ventricular (LV) function, and wide QRS complex. The abnormal activation sequence observed in patients with left bundle branch block (LBBB) results in a dyssynchronous ventricular activation and contraction leading to cardiac remodelling, worsening systolic and diastolic function, and progressive HF. The key concept of ‘biventricular pacing’ was developed with the aim to restore the dyssynchronous contraction resulting in improved symptoms, quality of life, exercise tolerance, cardiac function, and survival.1
Candidates’ selection: what guidelines tell us
Current guidelines recommend CRT in chronic HF patients with impaired cardiac function documented by LV ejection fraction (LVEF) ≤35% who remain in New York Heart Association (NYHA) function Class II, III, or ambulatory IV despite optimal medical therapy and typical LBBB with QRS duration ≥150 ms.2 Lower strength of recommendations appears when QRS duration is between 120 ms and 150 ms. Non-LBBB morphology should be considered only in patients with QRS duration ≥150 ms.2 Recently, sub-analyses of randomized clinical trials emphasized the primary role of QRS morphology over and above the QRS width showing a greater efficacy of CRT in patients with typical LBBB compared with patients with right bundle branch block (RBBB) or non-specific intraventricular conduction delay (IVCD).3,4
Even with well-selected patients, there is a wide range of response to CRT with a subset of patients showing little or no improvement.2 Since the early studies on the effects of conduction tissue disturbances on diastolic filling time and septal contribution to the LV ejection, the link between electrical dyssynchrony and mechanical contraction and cardiac output was clear.5,6 Therefore, echocardiography has been extensively tested to study mechanical dyssynchrony in order to identify the best parameters able to predict the efficacy of CRT, reducing the percentage of non-responders to the therapy. However, the recent PROSPECT (Predictors of Response to CRT) trial tested the efficacy of different echocardiographic measures of mechanical dyssynchrony but no one could reliably predict the response to CRT.7 The poor contribution of echocardiographic assessment of dyssynchrony for the prediction of CRT response was also confirmed by the EchoCRT trial that failed to show a benefit from CRT in patients with QRS ≤130 ms and dyssynchrony assessed echocardiographycally.8
Therefore, current guidelines recommend the use of QRS duration and morphology for the selection of HF patients as candidates for CRT. Left ventricular mechanical dyssynchrony assessed with imaging techniques is not currently considered a criterion for resynchronization therapy.2
Candidates’ selection: keep an eye on QRS duration and morphology
If the standard criteria used to identify HF patients with an LVEF ≤35% and a NYHA functional class between II and ambulatory IV is not under debate, the definition of complete LBBB has been extensively studied and discussed.
Under normal conditions, the myocardium is activated by a uniform, high-velocity electrical waveform that propagates through the His-Purkinje system and the bundle branches resulting in a synchronized depolarization of the ventricles. In patients with LBBB, ventricular activation starts in the right ventricle, because the right bundle branch is not affected, and then proceeds from the right ventricular endocardium to the LV endocardium through the interventricular septum. Then it propagates to the endocardium of the postero-lateral wall and it completely activates the ventricle without the use of the rapidly conducting Purkinje system. So in the presence of complete LBBB, there is a significant delay between the activation of the interventricular septum and the activation of the LV free wall, resulting in a QRS duration ≥140 ms9,10 (Figure 1).
Figure 1.

Electrical activation times and QRS duration in normal and complete left bundle branch block. Reprinted with permission from Strauss et al.9
Conventional electrocardiogram (ECG) criteria used clinically to describe LBBB morphology include: QRS duration ≥120 ms, QS or rS in lead V1 and a monophasic R wave with no Q waves in leads V6 and I. Defining complete LBBB, current guidelines recommend also to evaluate the presence of broad notched or slurred R wave in leads I aVL, V5 and V6 and an occasional RS pattern in V5 and V6 attributed to displaced transition of QRS complex.11 Notches or slurred R wave represent the propagation delay of the depolarization wave front to reach the endocardium of the left ventricle (first notch) and the epicardium of the postero-lateral wall (second notch) through the ventricular working myocardium instead of the rapidly-conducting Purkinje system.9
Several studies performed endocardial mapping in patients considered to have LBBB by conventional ECG criteria.12,13 It was demonstrated that almost one-third of the LBBB patients has two LV endocardial breakthrough sites instead of one, consistent with two of the three breakthrough sites described in normal hearts. In one-third of the LBBB patients, there is no significant delay between the right ventricular activation and the start of activation of LV endocardium with a transseptal time <20 ms suggesting that there is a subset of patients with an LBBB diagnosed by conventional criteria that do not actually have a complete LBBB but more likely a combination of left anterior fascicular block and LV hypertrophy.12,13
On the basis of additional insights from computer simulations, Strauss et al.9 proposed stricter criteria for complete LBBB that include mid-QRS notching or slurring in ≥2 contiguous leads and a QRS duration ≥140 ms for men and ≥130 ms for women. In a recent study, the presence of mid-QRS notching or slurring emerged as a strong predictor of better response to CRT.14
The initial randomized clinical trials, which led to the widespread use of CRT, selected patients only on the basis of QRS duration (≥120 ms) not focusing on QRS morphology. However, recent evidences emphasized the role of LBBB morphology in patients that underwent CRT. A report of Medicare registry showed that non-LBBB patients that received CRT had poorer outcomes compared to those with LBBB.15
Recent subgroup analyses based on QRS morphology of the MADIT-CRT, RAFT, and REVERSE trials suggested that patients with complete LBBB showed a greater benefit on the composite of morbidity/mortality from CRT compared with patients with RBBB or non-specific IVCD.4,16,17 In particular, in the MADIT-CRT, the use of CRT-defibrillator (CRT-D) in LBBB patients was associated with a clinical benefit compared with implantable cardioverter-defibrillator (ICD)-only therapy in all the pre-specified subgroups based on age, QRS duration ≥150 ms, LV volumes, and LVEF. No evidence of clinical benefit from CRT-D was identified in non-LBBB patients.4 A meta-analysis of the major CRT-trials confirmed these data suggesting that CRT implantation should be discouraged in non-LBBB patients.18 Therefore, based on this evidence, current Class I recommendations for CRT were restricted to patients with complete LBBB.
Candidates’s selection: right ventricular apical pacing for bradycardia and heart failure
In the last decade, increasing evidences showed that conventional right ventricular apical pacing might have detrimental effects on cardiac structure and LV function, possibly leading to the development of HF.19
The slow and heterogeneous propagation of the electrical wave front from the pacing site through the myocardium rather than through the His/Purkinje conduction system results in an abnormal activation pattern of the ventricles comparable to the LBBB. The mechanical activation pattern follows the changes in electrical activation showing an early systolic shortening of the regions near the pacing site with a resultant stretch of the late-activated regions. This abnormal contraction determines mechanical dyssynchrony, redistribution of myocardial strain, changes in cardiac metabolism and regional perfusion, decreased cardiac output, increased LV filling pressure, ventricular dilation, and functional mitral regurgitation. Several studies with a crossover design evaluated the upgrade from conventional pacemaker (PM) to CRT in patients requiring permanent or frequent right ventricular pacing for bradycardia who have symptomatic HF or low LVEF. In all of them, during CRT study phase, the patients consistently showed improved cardiac function, less hospitalization, symptoms’ improvement compared with the right ventricular study phase.20,21 Therefore, current guidelines strongly recommend the upgrade from conventional PM or ICD to CRT in all HF patients with LVEF <35%, high percentage of ventricular pacing who remain in NYHA Class III or more despite optimal medical therapy.2
In the PREventing VENTricular Dysfunction in Pacemaker Patients Without Advanced Heart Failure (PREVENT-HF) trial and in the biventricular vs. right ventricular pacing in patients with atrioventricular (AV) block (BLOCK-HF) trial, de novo CRT pacing was tested in patients with conventional indication for anti-bradycardia pacing. The PREVENT-HF showed no advantage to CRT compared with conventional right ventricular pacing in terms of LV remodelling in patients with AV block and expected ventricular pacing >80% after 12 months.7 In the BLOCK-HF trial, patients with AV block, LVEF ≤50%, and NYHA functional Class I to III were randomly assigned to biventricular or right ventricular pacing and followed for 37 months. The trial showed a significant reduction in the primary composite endpoint of death, HF-related urgent care or adverse LV remodelling in CRT patients compared with patients with right ventricular pacing only.22
Considering the observed detrimental effects of right ventricular pacing on LV function in patients with pre-existing LV dysfunction and high ventricular pacing rate, it was hypothesized that also patients with baseline normal cardiac function may be affected by pacing-induced mechanical dyssynchrony. However, in a large cohort of PM recipients, patients with AV block requiring frequent or permanent right ventricular pacing had similar survival with no difference in development of LV dysfunction or deterioration of pre-existing mild LV dysfunction after PM implantation compared with patients with sinus node dysfunction that required minimal right ventricular pacing.23 Preliminary results from the Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BIOPACE) trial showed no significant difference in the incidence of death and HF hospitalization after >5 years between biventricular pacing and right ventricular pacing in patients with conventional PM indication and preserved LV systolic function. Therefore, current guidelines recommend de novo CRT in HF patients with conventional PM indication, expected high percentage of ventricular pacing and reduced EF. At present, de novo CRT is not indicated in patients with baseline normal EF.
Candidates’ selection: keep an eye on PR interval
PR prolongation alters normal AV mechanical coupling reducing LV filling, stroke volume and resulting in diastolic mitral regurgitation. Dual-chamber pacing acutely improves haemodynamics restoring AV coupling but it failed to demonstrate improved long-term outcomes probably due to the detrimental effects of ventricular desynchronization. Therefore, patients with longer AV delay would be more likely to respond positively to CRT as it was described by a post hoc analysis of the COMPANION study.24
However, other studies have found that a prolonged PR interval seems to be a marker of atrial and structural remodelling and it is associated with more severe HF disease.25,26 The CARE-HF trial described worse outcomes in patients with prolonged PR interval regardless of the treatment arm (CRT or optimal medical therapy).27 In a recent study comparing patients with CRT, a baseline PR prolongation is an independent predictor of worse prognosis and lower probability of reverse remodelling, especially for patients with non-LBBB morphology.26
Conclusions
There is strong evidence that CRT reduces mortality and hospitalization and improves cardiac function in symptomatic HF patients despite optimal medical therapy with a depressed LVEF and complete LBBB. Recent evidences suggested that complete LBBB predicts better response to CRT therapy. Therefore, stricter criteria for LBBB that include wide QRS duration and mid-QRS notching or slurring in ≥2 contiguous leads should be used in order to identify the true LBBB configuration (Figure 2).
Figure 2.
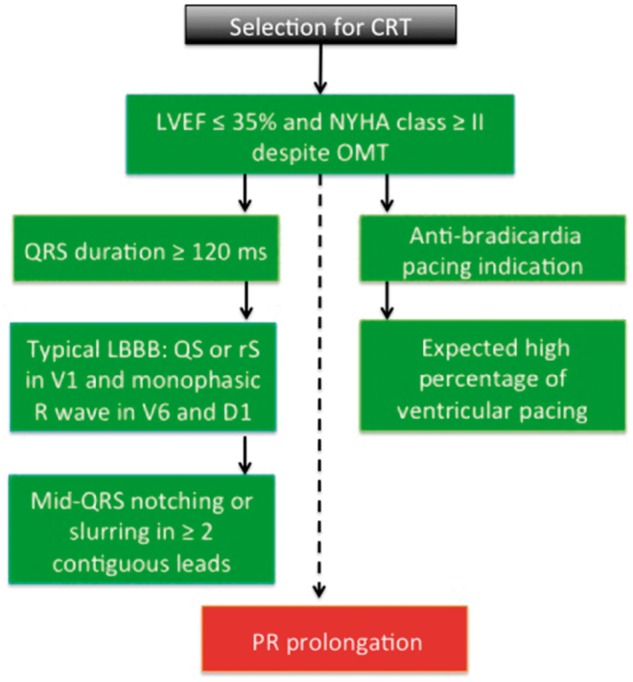
Schematic representation of clinical and electrocardiographic characteristics of patients before cardiac resynchronization therapy. CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OMT, optimal medical therapy.
Right ventricular apical pacing might have long-term deleterious effects on cardiac structure and function. Therefore, current guidelines recommend upgrade from conventional PM or ICD to CRT or de novo CRT in patients with high (or expected high) percentage of ventricular pacing and reduced EF (Figure 2).
A prolonged PR interval seems to be a marker of atrial and structural remodelling and it is an independent predictor of worse prognosis and lower probability of reverse remodelling after CRT (Figure 2).
Conflict of interest: none declared.
References
- 1. Leyva F, Nisam S, Auricchio A.. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol 2014;64:1047–1058. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE; ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in Collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 3. Birnie DH, Ha A, Higginson L, Sidhu K, Green M, Philippon F, Thibault B, Wells G, Tang A.. Impact of QRS morphology and duration on outcomes after cardiac resynchronization therapy: results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail 2013;6:1190–1198. [DOI] [PubMed] [Google Scholar]
- 4. Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ.. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011;123:1061–1072. [DOI] [PubMed] [Google Scholar]
- 5. Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P, Wooley CF.. Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation 1989;79:845–853. [DOI] [PubMed] [Google Scholar]
- 6. Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS.. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol 1990;259:H300–H308. [DOI] [PubMed] [Google Scholar]
- 7. Stockburger M, Gómez-Doblas JJ, Lamas G, Alzueta J, Fernández-Lozano I, Cobo E, Wiegand U, de la Concha JF, Navarro X, Navarro-López F, de Teresa E.. Preventing ventricular dysfunction in pacemaker patients without advanced heart failure: results from a multicentre international randomized trial (PREVENT-HF). Eur J Heart Fail 2011;13:633–641. [DOI] [PubMed] [Google Scholar]
- 8. Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, Dickstein K, Ford I, Gorcsan J, Gras D, Krum H, Sogaard P, Holzmeister J.. Echo CRTSG. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. New Engl J Med 2013;369:1395–1405. [DOI] [PubMed] [Google Scholar]
- 9. Strauss DG, Selvester RH, Wagner GS.. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol 2011;107:927–934. [DOI] [PubMed] [Google Scholar]
- 10. van Stipdonk A, Wijers S, Meine M, Vernooy K.. ECG patterns in cardiac resynchronization therapy. J Atr Fibrillation 2015;7:1214.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:976–981. [DOI] [PubMed] [Google Scholar]
- 12. Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, Kloss M, Klein H.. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation 2004;109:1133–1139. [DOI] [PubMed] [Google Scholar]
- 13. Vassallo JA, Cassidy DM, Miller JM, Buxton AE, Marchlinski FE, Josephson ME.. Left ventricular endocardial activation during right ventricular pacing: effect of underlying heart disease. J Am Coll Cardiol 1986;7:1228–1233. [DOI] [PubMed] [Google Scholar]
- 14. Tian Y, Zhang P, Li X, Gao Y, Zhu T, Wang L, Li D, Wang J, Yuan C, Guo J.. True complete left bundle branch block morphology strongly predicts good response to cardiac resynchronization therapy. Europace 2013;15:1499–1506. [DOI] [PubMed] [Google Scholar]
- 15. Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ.. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation 2010;122:2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL; Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. New Engl J Med 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 17. Gold MR, Thébault C, Linde C, Abraham WT, Gerritse B, Ghio S, St John Sutton M, Daubert J-C.. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation 2012;126:822–829. [DOI] [PubMed] [Google Scholar]
- 18. Sipahi I, Chou JC, Hyden M, Rowland DY, Simon DI, Fang JC.. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Am Heart J 2012;163:260–267.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ, Hellkamp AS, Greer S, McAnulty J, Ellenbogen K, Ehlert F, Freedman RA, Estes NAM, Greenspon A, Goldman L; Mode Selection Trial in Sinus-Node Dysfunction. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. New Engl J Med 2002;346:1854–1862. [DOI] [PubMed] [Google Scholar]
- 20.Leclercq C, Cazeau S, Lellouche D, Fossati F, Anselme F, Davy JM, Sadoul N, Klug D, Mollo L, Daubert JC. Upgrading from single chamber right ventricular to biventricular pacing in permanently paced patients with worsening heart failure: the RD-CHF Study. Pacing Clin Electrophysiol 2007;30(Suppl 1):S23–S30. [DOI] [PubMed] [Google Scholar]
- 21. van Geldorp IE, Vernooy K, Delhaas T, Prins MH, Crijns HJ, Prinzen FW, Dijkman B.. Beneficial effects of biventricular pacing in chronically right ventricular paced patients with mild cardiomyopathy. Europace 2010;12:223–229. [DOI] [PubMed] [Google Scholar]
- 22. Curtis AB, Worley SJ, Chung ES, Li P, Christman SA, St John Sutton M.. Improvement in clinical outcomes with biventricular versus right ventricular pacing: the BLOCK HF study. J Am Coll Cardiol 2016;67:2148–2157. [DOI] [PubMed] [Google Scholar]
- 23.Micaela Ebert, Nikolaus Jander, Jan Minners, Thomas Blum, Michael Doering, Andreas Bollmann, Gerhard Hindricks, Thomas Arentz, Dietrich Kalusche, Sergio Richter. Long-term impact of right ventricular pacing on left ventricular systolic function in pacemaker recipients with preserved ejection fraction: results from a large single-center registry. J Am Heart Assoc 2016;5:e003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin J, Buhr KA, Kipp R.. Effect of PR interval on outcomes following cardiac resynchronization therapy: a secondary analysis of the COMPANION trial. J Cardiovasc Electrophysiol 2017;28:185–191. [DOI] [PubMed] [Google Scholar]
- 25. Januszkiewicz Ł, Vegh E, Borgquist R, Bose A, Sharma A, Orencole M, Mela T, Singh JP, Parks KA.. Prognostic implication of baseline PR interval in cardiac resynchronization therapy recipients. Heart Rhythm 2015;12:2256–2262. [DOI] [PubMed] [Google Scholar]
- 26. Rickard J, Karim M, Baranowski B, Cantillon D, Spragg D, Tang WHW, Niebauer M, Grimm R, Trulock K, Wilkoff B, Varma N.. Effect of PR interval prolongation on long-term outcomes in patients with left bundle branch block vs non-left bundle branch block morphologies undergoing cardiac resynchronization therapy. Heart Rhythm 2017;14:1523–1528. [DOI] [PubMed] [Google Scholar]
- 27. Gervais R, Leclercq C, Shankar A, Jacobs S, Eiskjaer H, Johannessen A, Freemantle N, Cleland JGF, Tavazzi L, Daubert C; CARE-HF investigators . Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail 2009;11:699–705. [DOI] [PubMed] [Google Scholar]


