Abstract
Importance. Oncology trials often entail high-stakes interventions where potential for morbidity and fatal side effects, and for life-prolongation or cure, intensify bioethical issues surrounding informed consent. These challenges are compounded in multistage randomized trials, which are prevalent in oncology. Objective. We sought to elucidate the major barriers to informed consent in high-stakes oncology trials in general and the best consent practices for multistage randomized trials. Evidence Review. We queried PubMed for original studies published from January 1, 1990, to April 5, 2018, that focused on readability, quality, complexity or length of consent documents, motivation and sickness level of participants, or interventions and enhancements that influence informed consent for high-stakes oncologic interventions. Exclusion criteria included articles focused on populations outside industrialized countries, minors or other vulnerable populations, physician preferences, cancer screening and prevention, or recruitment strategies. Additional articles were identified through comprehensive bibliographic review. Findings. Twenty-seven articles were retained; 19 enrolled participants and 8 examined samples of consent documents. Methodologic quality was variable. This body of literature identified certain challenges that can be readily remedied. For example, the average length of the consent forms has increased 10-fold from 1987 to 2010, and patient understanding was shown to be inversely proportional to page count; shortening forms, or providing a concise summary as mandated by the revised Common Rule, might help. However, barriers to understanding that stem from deeply ingrained and flawed sociocultural perceptions of medical research seem more difficult to surmount. Although no studies specifically addressed problems posed by multiple sequential randomizations (such as change in risk-benefit ratio due to time-varying treatment responses or organ toxicities), the findings are likely applicable and especially relevant in that context. Concrete suggestions for improvement are proposed.
Keywords: Informed consent, randomized trials, oncology, multistage trials
The informed consent process was designed to impart understanding of the purpose of a given research study, as well as its potential risks, benefits, inconveniences, and alternative experimental and standard-of-care options. Oncology trials often entail high-stakes interventions, where the potential for morbidity and for fatal side effects, as well as for life-prolongation or cure, intensify bioethical issues related to informed consent. Many factors influence the ability of a patient to understand the proposed trial. Some of these are germane to all clinical trials, such as readability, complexity, length, and quality of the informed consent forms (ICFs; Table 1). While these issues are amplified in multistage randomized clinical trials (RCTs), these trials also impose unique challenges on the informed consent process. Multistage RCTs are common in oncology (Table 2) and are an important way to advance cancer research and ultimately improve patient outcomes.1,2 In these complex trials, patients are first randomized between initial treatment options, and then randomized again, at one or more serial time points, depending on their responses to the treatments. In this article, we examine the approaches to informed consent currently recommended by bioethicists and medical communication specialists. We highlight where these recommendations conflict. Although no studies directly addressed the optimal consent process for multistage randomized trials, we discuss the ramifications of this body of literature for this increasingly prevalent oncologic trial design. We urge bioethicists to consider the unique features of multistage RCTs in future research on consent. The present article is not intended to question the ideal of informed consent as a professionally sanctioned, coercion-free process leading to thorough understanding on the part of the patient; it is intended to offer some insights about why the process often falls short in practice, and how we could facilitate realization of this ideal.
Table 1.
Variables That Influence Understanding of Informed Consent Forms (ICFs)
| Variable | Definition | Findings |
|---|---|---|
| Readability | Reading ease and reading grade level | Studies on the readability of ICF were mixed but one study indicated readability did not translate to better understanding. |
| Simplicity | Succinct explanations, familiar words, short sentences, plain language, use of bullet points, and diagrams | Participants preferred simplified forms, but results on whether understanding improved were mixed. |
| Length | Word or page count | Understanding is inversely proportional to length. |
| Quality | All components of consent adequately represented and explained | Components most likely to be absent or minimized include the investigational nature of the trial, the scientific basis for the trial, and the anticipated benefit to self versus others. |
| Stakes involved | Potential for benefit or harm as determined by the sickness level and outlook of participants recruited to study and the riskiness versus curative potential of the proposed treatments | The sicker the patients, the lower their understanding and the greater the prevalence of the “therapeutic myth.” |
Table 2.
Examples of Sequentially Randomized Trials in Oncology
| Disease (Reference) | N | First Randomization | Eligibility Criteria for Second Randomization | Second Randomization |
|---|---|---|---|---|
| Non–small cell lung cancer (Belani et al., 2003)33 | 401 | Three different schedules/dose-levels of paclitaxel and carboplatin | Adequate response at week 16 | Paclitaxel continuation therapy versus observation |
| Neuroblastoma (Matthay et al., 2009)34 | 379 | Before the last induction cycle, patients were randomized to one of two consolidation options: autologous purged bone marrow transplantation conditioned with myeloblative chemotherapy and total body irradiation versus three cycles of intensive chemotherapy. | Completion of consolidation with absence of disease progression | 13-cis-retinoic acid × 6 cycles versus observation |
| Metastatic prostate cancer (Thall et al., 2007)35 | 150 | One of four chemotherapy regimens (CVD versus KA/VE versus TEC versus TEE) | Nonresponse (evaluated every 8 weeks) | One of the remaining three chemotherapy regimens to which the patient was not already exposed |
| Multiple myeloma (Mateos et al., 2010)36 | 260 | Induction with one of 2 regimens: VMP versus VTP | Completion of six induction cycles | Maintenance therapy with bortezomib + prednisone versus bortezomib + thalidomide |
| Relapsed or refractory aggressive non-Hodgkin B-cell lymphoma (Kuruvilla et al., 2013, and Crump et al., 2014)37,38 | 554 | One of two chemotherapy regimens: GDP versus DHAP | Recovery from autotransplant toxicity and progression-free status at 3 to 5 weeks post-transplant | Maintenance rituximab versus observation |
| Untreated diffuse large B cell lymphoma (Habermann et al., 2006)39 | 632 | Chemotherapy (CHOP) versus chemo-immunotherapy (R-CHOP) | Adequate response | Maintenance rituximab versus observation |
| Relapsed/resistant follicular lymphoma(van Oers et al., 2006)40 | 465 | Chemotherapy (CHOP) versus chemo-immunotherapy (R-CHOP) | Adequate response | Maintenance rituximab versus observation |
| Acute myeloid leukemia (Stone et al., 2001)41 | 388 | GM-CSF versus placebo added to standard induction chemotherapy | Morphologic complete remission and fitness for further therapy | One of two consolidation regimens: cytarabine versus cytarabine + mitoxantrone |
| Multiple myeloma, ongoing as of 2017 (ECOG-1A11 ENDURANCE, NCT01863550) | Target: 756 | Induction with 12 cycles of VRd versus 9 cycles of CRd | No progression on stage 1 therapy | Maintenance lenalidomide for 2 years versus indefinitely |
| Advanced renal cell cancer, ongoing as of 2017 (NCT01217931) | Target: 240 | Pazopanib versus everolimus versus bevacizumab | No response | Randomized between the two agents not yet used |
| CLL (Matutes et al., 2013)42 | 777 | Chlorambucil or fludarabine alone versus with cyclophosphamide | No response, progression, or relapse within 1 year of remission | Protocol-guided chemotherapy regimen versus TRAC assay-guided regimen |
| Locally advanced pancreatic cancer (Hammel et al., 2016)43 | 223 | Gemcitabine alone versus gemcitabine + erlotinib | Progression-free after 4 months | Two months of the same chemotherapy versus capecitabine + 54 Gy radiotherapy |
| Metastatic colorectal cancer (Tournigand et al., 2015)44 | 156 | mFOLFOX7 + bevacizumab versus XELOX2 + bevacizumab | No progression after 3 months | Maintenance bevacizumab versus bevacizumab + erlotinib |
CRd, Carfilzomib, Revlimid (lenalidomide), dexamethasone; CVD, cyclophosphamide, vincristine, dexamethasone; DHAP, dexamethasone, high-dose cytarabine, cisplatin; GDP, gemcitabine, dexamethasone, cisplatin; GM-CSF, granulocyte colony-stimulating factor; KA/VE, ketoconazole + doxorubicin/vinblastine + estramustine; mFOLFOX7, modified folinic acid, fluorouracil, oxaliplatin; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; TEC, paclitaxel, estramustine, carboplatin; TEE, paclitaxel, estramustine, etoposide; TRAC, tumor response to anti-neoplastic compounds; VMP, Velcade (bortezomib), melphalan, prednisone; VRd, Velcade (bortezomib), Revlimid (lenalidomide), dexamethasone; VTP, Velcade (bortezomib), thalidomide, prednisone; XELOX2, biweekly capecitabine (Xeloda), oxaliplatin.
Literature Search Strategy
We queried the PubMed database on April 5, 2018, as follows: ((informed consent[MeSH Major Topic]) AND clinical trials[MeSH Major Topic]) NOT children[MeSH]. We applied the subject filter for “cancer” and the publication date range of January 1, 1990, to April 5, 2018. This search retrieved 189 articles. We then reviewed the titles and abstracts for relevance. Inclusion criteria were original studies on readability, quality, simplicity, length and complexity of consent documents, motivation and sickness level of participants, as well as consent interventions and enhancements. Exclusion criteria included articles not in English, articles that focused on populations outside industrialized countries, articles that focused on minors or other vulnerable populations, articles on physician preferences, articles about cancer screening and prevention trials, articles that focused on recruitment strategies but not specifically on the consent process, and articles not related to high-stakes oncologic interventions. Specifically, articles related to surgery, radiation therapy, chemotherapy, immunotherapy, targeted anticancer drugs or antibodies, and stem cell transplantation were included; articles related to registry enrollment or biomarker monitoring were not. Seventeen articles met our eligibility criteria. We searched the bibliographies of the 17 selected articles to identify another 236 potentially relevant articles, of which 10 met our criteria and were selected. These 27 articles form the basis of this narrative review. The assessment of methodologic quality is depicted in eTable 1.
Patient Understanding
Patients do not comprehend many elements of informed consent.3 Participants in clinical trials often have a difficult time grasping the difference between research and treatment goals. They may believe the “therapeutic myth” that the intention of the trial is to find a treatment or even a cure for the actual participants in the trial. For instance, Joffe and colleagues reported that 30% of research participants in oncology trials believe that the therapy being evaluated in the trial was already proven to be the most effective treatment.3 In another study, Behrendt and colleagues aimed to evaluate 13 different categories regarding patients’ comprehension of informed consent. Interviewing patients after they had signed the informed consent documents, researchers found a distinct lack of knowledge about the contents of the document, about the RCT process, and especially about the experimental aspect of the trial. Participants were, for the most part, eager to help with the research process but held tight to the belief that the trial could benefit them as well.4 A 2003 study of lung cancer patients across 44 institutions also showed a substantial lack of knowledge regarding the benefit to self versus others; two thirds believed that the purpose of the study was to treat their own cancer.5 It is important to identify the elements of the informed consent process that undermine the ability to legally and ethically elicit true informed consent from participants by imparting to them the equipoise or experimental nature inherent in the research. This dilemma is magnified in multistage trials—where participants are subjected to multiple randomizations—because the sequential randomizations may each have a different degree of “novelty” and vastly different risks or potential benefits. Also, participants may have a degree of misunderstanding of terminology associated with newer therapies.
For example, there is a notion prevalent among cancer patients that “targeted therapies” and “immunotherapies” are somehow less toxic than traditional chemotherapy.6 It may be important to overtly address that mistaken notion when administering informed consent for such novel therapies.
Readability of Informed Consent Forms
Readability, as defined by reading grade level and reading ease of the informed consent document, has been considered an important factor in participant understanding. This assumption is based on the fact that more than 50% of American adults read below eighth-grade level,7 which is substantially below the level of readability of most consent documents. In 1994, Grossman and colleagues assessed 137 consent forms from 88 clinical trial protocols at John Hopkins Oncology Center. They evaluated the consent documents with the Flesch-Kincaid Formula and reported a mean reading grade level of 11.1. Only 6% of the consent forms scored at the eighth-grade level or below. They concluded that the reading level of the consent documents would make them difficult for the majority of patients and their families to comprehend.8 A 2010 study by Cheung and colleagues did not confirm the Grossman study and reported a significantly lower reading grade level in the consent documents for 262 oncology trials approved by the University Health Network Research Ethics Board in Toronto. Using the Flesch-Kincaid Reading Grade Level, the average reading grade level of the documents was found to be 7.4.9 However, in 2017 Schumacher and colleagues reported findings similar to Grossman and colleagues. They analyzed the ICFs for 26 clinical studies at Brown University Oncology Research Group and found a mean reading level of 11.7 and a mean Reading Ease Score of 50.2, indicating the reading level was between “fairly difficult” and “difficult.”10 These conflicting results may be due to variations in ICF approval policies between institutional review boards (IRBs), or to the mix of industry and cooperative group trials compared with institutional trials in the different samples, as institutional IRBs may have less ability to effect modifications to consent forms that originate from pharmaceutical companies or cooperative groups, especially for multisite studies.
The focus on readability arises because of our intuition that improving readability ought to improve patients’ understanding. However, evidence suggests that improved readability does not necessarily result in improved understanding. Coyne and colleagues compared standard consent forms with forms adapted for readability across 44 institutions and found that although patient anxiety decreased and satisfaction increased, the easy-to-read forms did not change the comprehension level of the participants.5
Complexity
The simplicity of the document or alternatively, the complexity of the document, may also be a factor in the understanding of ICFs, but results from various studies are mixed. Simplified consent documents are characterized by short familiar words, short sentences, plain language, and bullet point format. Diagrams are often used as well. In fact, graphics were used in the simplified consent forms in both a 1998 and a 2015 study. The 1998 study aimed to determine if a simplified ICF would be easier to understand than a more complex one. The authors found that though participants greatly preferred the simplified form, comprehension was nearly identical between the two documents.11 However, the 2015 study found just the opposite. One hundred and fifty participants were randomized to either a standard or simplified consent form for an oncology clinical trial. Participants had a much higher level of understanding with the simplified form, and this was noted across all levels of health literacy.12 Perhaps the different findings between the two studies can be attributed to the increase in complexity of the standard consent form in the 17-year time lapse between the conduct of these studies (as simplification may show a greater effect if the “control” form is more complicated), or to the higher proportion of college-educated patients in the 1998 study (∼50% v. 35%). Differences between the populations in baseline health literacy and in familiarity with clinical trials are difficult to assess because different instruments were used to measure these attributes, but these factors may also account for the observed effects. The value of presenting information in a simple format may become increasingly beneficial as patients are less familiar with the clinical trial design, as treatment itself becomes more complex and as the consent process becomes more involved—as is the case for trials that entail multiple randomization stages.
Quality
The quality of explanations and inclusion of all elements of consent also influences participant comprehension of the nature and benefits of a clinical trial. In a survey of 207 patients involved in oncology trials, Joffe and colleagues aimed to measure “quality of informed consent” as a function of patient satisfaction as well as patient comprehension of the informed consent process. They developed a quality of informed consent (QuIC) questionnaire and found that a full 90% of the patients identified themselves as both satisfied and well-informed. However, many of the same participants did not understand key factors of the consent such as the potential for harm, the experimental nature of treatment, and the fact that benefits to self may not occur. Only 46% recognized that the goal of the trial was to benefit future patients.3 Another study using the QuIC questionnaire to assess participants’ actual understanding versus perceived understanding found both patient satisfaction and patient comprehension were high. Despite that, the study also found that key elements of consent regarding the benefits to self versus the benefits to future patients were often missing from ICFs, perpetuating the belief that the purpose of the study is therapeutic.13 One study of ICFs from neuro-oncology RCTs found that only 33% addressed the scientific background of the proposed experimental treatments.14
Length
Length also affects participant understanding of informed consent documents. It is measured by word or page count. The mean length of the ICF has increased 10-fold in the last three decades (Figure 1).9,15 In one study, the average length of informed consent documents was 338 words in 1987 and 1087 words in 2005,15 while in 2010, a review of the ICFs for 262 oncology clinical trials showed an average length of 3982 words.9 A review of 9 ICFs for multicenter phase III RCTs conducted at the German Brain Tumor Center in 2011–2012 found an average length of 19 pages (range 12–30) and average word count of 7069 words.14 The reasons for the increase in ICF length are not well defined in these studies. We speculate that fear of litigation might lead regulatory bodies to demand legally precise jargon and comprehensive risk descriptions. However, such ICFs fail to convey the distinction between material risks and extremely unlikely ones. At the same time, patient understanding has decreased and is inversely proportional to the page count of the informed consent document.13 Beardsley and colleagues evaluated knowledge and satisfaction of participants in 27 clinical trials and found that the level of knowledge was inversely proportional to the length of the ICF. The study found that the objective knowledge score (QuIC-A) on the QuIC was substantially higher when the page count of the document was seven or less.13
Figure 1.
The mean number of words in informed consent forms from 1987 to 1989 was 338 (SD 67), from 2005 to 2007 it was 1087 (SD 594), and from 2009 to 2010 it was 3982 (SD 1320). Error bars show the standard deviations (SD).
One striking feature about ICFs is the redundancy from form to form of paragraphs that deal with protection of confidentiality, dissemination of anonymized information in scientific presentations and publications, publication on ClinicalTrials.gov, inspection of medical and research charts by trial sponsors and regulatory bodies, and lack of financial compensation of participants. If this information could be separated from the ICF about the trial at hand (assuming it is applicable to that trial), it could be placed online, in booklets around the clinic, and included in the packet of initial or annual intake forms. Then, the “General Information about Participation in Clinical Trials” could be referenced, but not wholly copied, into the consent forms for the relevant individual trials, leaving the focus of that form on the decision about whether to accept an experimental treatment, the trial design, and what would be expected from the participant. A separate signature section on the trial’s ICF could attest to whether the patient read and understood the “General Information” that was provided separately. Legal frameworks and IRB policies that allow for such an approach would need to be developed. Current IRB policies seem to result in the opposite effect. For example, one study of 197 consent forms from 56 cooperative group trials found that templates supplied by cooperative groups are often expanded-upon by local IRBs, from a mean of 13 to 17 pages.16 The revised Common Rule, section 116(a)(5)(i), now requires
that informed consent must begin with a concise and focused presentation of the key information that is most likely to assist a prospective subject or legally authorized representative in understanding the reasons why one might or might not want to participate in the research. This provision further mandates that this part of the informed consent must be organized and presented in a way that facilitates comprehension.
The length of the “concise and focused” preamble is not specified in the revised Common Rule. These changes will become effective January 21, 2019. It will be important for bioethicists to measure their impact on the success of the informed consent process.
High Stakes
Oncology trials often involve participants who are very sick and who require dramatic treatments like surgery, radiation, chemotherapy, or immunotherapy. The stakes are high because of the potential for morbidity and for fatal side effects, as well as the potential for life-prolongation or cure. The anxiety over high-stakes interventions may affect ability to understand the consent documents and may also magnify other factors impacting consent. Schaeffer and colleagues aimed to determine if the severity of a participant’s illness is a major factor in the understanding of informed consent documents. They reported that seriously ill patients experienced more stress and were less likely to comprehend risks of treatment, viewing themselves as patients seeking treatment rather than as research participants.17 Bergenmar and colleagues did not find a correlation between type of cancer (breast, gastrointestinal, lung, melanoma, urologic, gynecologic, etc.) and understanding of the consent forms.18 Unfortunately, they did not specifically measure the “sickness” level of the participants or report the cancer stage. They also focused on phase 2 and 3 clinical trials so patients with the more severe illnesses (and therefore more anxiety), who might more likely be directed to phase I trials, may have been underrepresented.
Interventions to Improve Informed Consent
Though results are mixed, on balance, enhancement of informed consent documents by improving readability, redesigning forms with the goal of simplicity, limiting the length of the documents, and monitoring carefully for inclusion of all elements of consent seem to modestly improve patient comprehension. Table 3 lists additional modifications to the consent process that have been shown in studies to not only improve patient understanding but also increase satisfaction and decrease anxiety. Decision aids are one such intervention. They include booklets, brochures, and patient information sheets that are designed to support patients’ decision making. For instance, Campbell and colleagues designed a clinical trials information handbook and found that individuals who reviewed the handbook scored 80% higher on understanding than the control group.19 Another study provided a staged set of information sheets for advanced colorectal cancer patients and found that 90% to 95% of the patients reported full or almost-full understanding of the content of the informed consent documents.20
Table 3.
Interventions to Improve Informed Consent
| Medium | Examples |
|---|---|
| Decision aids | • Booklets |
| • Brochures | |
| • Staged information sheets | |
| Multimedia | • Audiovisual presentations |
| • Animated videos | |
| • Slide show with voice-over | |
| • DVD/computer | |
| Computerized information | • Interactive software on computer or tablet |
| • Web-based applications | |
| • Patient-driven | |
| • Set up to test for knowledge | |
| Personal discussion | • Communication-trained physician |
| • Communication-trained patient advocate | |
| Miscellaneous | • Patient “repeat back” |
| • Patient testing | |
| • Time for contemplation between receipt of information and signing | |
| • Incentives | |
| • Shorten consent forms |
Multimedia constitutes another category of useful interventions. Both Hutchison and colleagues21 and Kraft and colleagues22 found that using audiovisual aids to explain clinical trials improved patient knowledge and understanding. Additionally, computer-based technology holds promise as a means to enhance the informed consent process. In 2009, Tait and colleagues compared patient understanding of interactive-computerized information to standard printed information and found that understanding was much higher for the interactive-computerized arm.23 A 2013 study found that an interactive, tablet-based informed consent module led to substantially higher understanding compared with standard paper consents. The computer consent group scored higher on every quiz question. The computerized information delivery did not appear to affect participation rates: 62% of the participants given the computerized consent opted to participate in the study compared to 69% of the paper-based subjects (P = NS).24 Another study by Kass and colleagues compared early-phase oncology trial participants who were randomized to either receive a pre-consent brochure or to receive a computer-based aid. While they reported that the computer-based model was able to alter understanding about purpose and benefit of the clinical trial, many respondents still held to the belief that the clinical trial offered them a cure.25
Studies found that repeat backs26 and monetary incentives27 can enhance recall of elements of informed consent. Finally, discussion with a communication-trained physician or patient advocate shows promise in increasing patient understanding of the clinical trial, allowing for a high level of informed consent.28
Effective communication requires general skills as well as an awareness of the specific barriers to understanding posed by the trial concerned. For example, the landmark ProtecT study approached 2664 men with localized prostate cancer, of whom 1643 (62%) agreed to randomization to one of three arms (monitoring, radical prostatectomy, or radiotherapy). An embedded quality improvement study by Donovan and colleagues conducted interviews with men after receipt of the diagnosis to elicit their baseline treatment preferences, analyzed audiotapes of the consent appointments (and other medical appointments), and interviewed the men after the consent appointments to examine the delivery of information and its interpretation by patients.29 Misunderstandings arose from recruiters’ use of medical jargon not understood by patients—including using the term “trial” instead of “study”—or confusing presentation of survival/mortality statistics. Avoiding jargon and presenting statistics in a readily digestible format are teachable and broadly applicable skills. The quality improvement (QI) team also identified barriers specific to understanding the ProtecT study, namely, communicating what “monitoring” actually entailed (as it was often interpreted as “the doctor will watch me die”) and communicating the equipoise that existed for all three arms (because recruiters were themselves biased toward the interventional arms and tended to spend little time on the monitoring option). At three points in time, the QI team circulated documents to the study sites suggesting how best to conduct the consent meetings based on their interim findings, and at a fourth and final time point, they intensively trained recruiters. These interventions improved the consent to randomization from 30% to 40% to 70% over 1 year.
Implications for Multistage Clinical Trials
In a multistage clinical trial with two (or more) randomizations, there may effectively be four (or more) research arms as opposed to the two in a traditional single-stage randomized trial (Figure 2). Issues of informed consent including readability, complexity, length, and quality of documents are magnified by multistage clinical trials since informed consent must be achieved for each randomization. For example, the issue of length is pertinent to multistage trials because if the patient is to be presented with all treatment options up-front, that mandates inclusion of the risks, benefits, and alternatives for each treatment option, which substantially prolongs the consent form and process. Presenting such patients with treatment options up-front also means that “superfluous” information may be included. For example, if subsequent randomizations are open only to responders, a patient who is destined to be a nonresponder does not necessarily need to learn all the details about treatment options available only to future responders. However, without a crystal ball or some source of omniscience, it is not possible to know who will and who will not respond. Also, ethically, patients need to know that they are consenting to a multistage trial, so need some information about what each of those stages comprises.
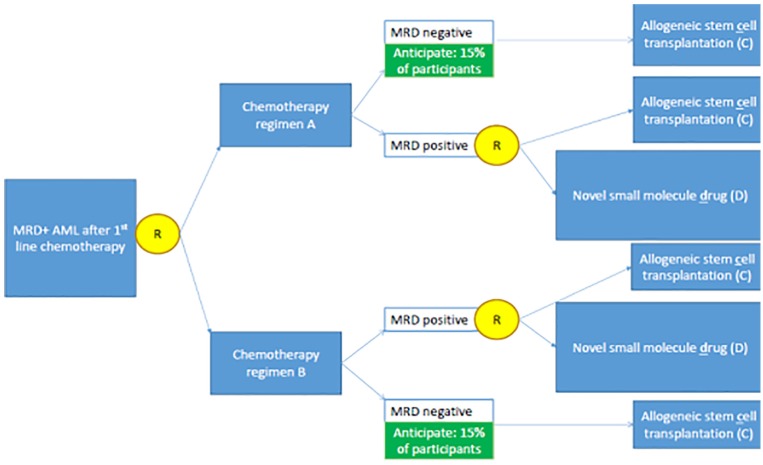
Figure 2.
Example of a multistage randomized trial where sequential consent or re-consent might be appropriate. Yellow circles refer to the randomizations. This trial examines whether patients who receive induction chemotherapy for acute myeloid leukemia (AML) and are then found to have measurable residual disease (MRD) should receive chemotherapy regimen A or B in an attempt to eradicate MRD. Nonresponders (i.e., persistently MRD-positive patients) are then randomized to allogeneic stem cell transplantation (C) or an experimental small molecule drug (D). All options have the potential for life prolongation or cure, but also carry very different and serious risks. Therefore, they are “high stakes” interventions. Patients may experience different trajectories: (1) A → MRD-negative → C, (2) A → MRD-positive → D, (3) B → MRD-negative → C, (4) B → MRD-positive → D. Complicating the consent process is the fact that allogeneic stem cell transplantation carries drastically different risks of cancer relapse for patients who enter transplant in an MRD-positive versus MRD-negative state, and the small molecule drug has a vastly different risk of cardiotoxicity for patients who previously received chemotherapy regimen A compared with those who previously received chemotherapy regimen B.
Given the general lack of patients’ comprehension of the trial purpose and process, as well as the possible changes in clinical or psychosocial status between phases of a multistage randomized trial that might dynamically influence the risk-benefit calculation of entering the next stage, it may be advisable to re-consent participants in multistage randomized trials prior to each randomization. However, the consent process is already a deterrent to clinical trial participation.30,31 The need to re-consent may cause trial dropout and participant anxiety, so phasing the consent could also prove problematic.32 Re-consenting also risks differential drop-out (e.g., sicker subjects or those who experienced a treatment-emergent adverse event may be less likely to re-consent), which could introduce selection bias as the study progresses through sequential randomizations. These questions merit dedicated qualitative and interventional research, such as that performed within the ProtecT study described in the previous section.
To address the dilemma of consent for multistage trials, it will be essential to find what combination of document enhancement and interventions best foster patient understanding. An example of this would be a hybrid interactive computer program to explain the goal and investigational nature of the clinical trial, a diagrammatic consent document as an overview of all arms prior to enrollment, and simplified “snapshot” aids to re-consent to a specific randomization as it becomes available based on the patient’s response in real-time.
Conclusion
Informed consent documents for clinical trials are often lengthy, poorly readable, complex, and lead to a process of questionable quality. Participants recruited to multistage oncology trials are often seriously ill, may have preexisting misconceptions about research, and face high-stakes medical treatments. Poor patient understanding can be an unfortunate by-product of these factors. Such factors are likely even more problematic for multistage trials. Although multistage randomized trials are common in oncology, there is a lack of literature on which consent processes most suit them. Is a onetime comprehensive consent process sufficient or is it necessary to remind patients as they progress through the study of its investigational nature, and to formally review the risk-benefit balance of the upcoming treatment options before each subsequent randomization? Can modern approaches to enhancing the consent process improve patient satisfaction and comprehension? We encourage researchers to include consent-related aims in the design of their multistage trials so that our field can better fulfill both the legal and ethical requirements of informed consent.
Supplemental Material
Supplemental material, Online_Supplement_online_supp for The Challenges of Informed Consent in High-Stakes, Randomized Oncology Trials: A Systematic Review by Julia M. Nathe and Elizabeth F. Krakow in MDM Policy & Practice
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at https://journals.sagepub.com/home/mpp.
Contributor Information
Julia M. Nathe, School of Medicine
Elizabeth F. Krakow, Division of Medical Oncology; University of Washington, Seattle, Washington; Division of Clinical Research, Fred Hutchinson Cancer Research Center, Seattle, Washington.
References
- 1. Kidwell KM. SMART designs in cancer research: past, present, and future. Clin Trials. 2014;11(4):445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kosorok MR, Moodie EEM. Adaptive Treatment Strategies in Practice: Planning Trials and Analyzing Data for Personalized Medicine. Philadelphia: Society for Industrial and Applied Mathematics; 2016. [Google Scholar]
- 3. Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001;358(9295):1772–7. [DOI] [PubMed] [Google Scholar]
- 4. Behrendt C, Gölz T, Roesler C, Bertz H, Wünsch A. What do our patients understand about their trial participation? Assessing patients’ understanding of their informed consent consultation about randomised clinical trials. J Med Ethics. 2011;37(2):74–80. [DOI] [PubMed] [Google Scholar]
- 5. Coyne CA, Xu R, Raich P, et al. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21(5):836–42. [DOI] [PubMed] [Google Scholar]
- 6. Reeder-Hayes KE, Roberts MC, Henderson GE, Dees EC. Informed consent and decision making among participants in novel-design phase I oncology trials. J Oncol Pract. 2017;13(10):e863–e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis TC, Williams MV, Marin E, Parker RM, Glass J. Health literacy and cancer communication. CA Cancer J Clin. 2002;52(3):134–49. [DOI] [PubMed] [Google Scholar]
- 8. Grossman SA, Piantadosi S, Covahey C. Are informed consent forms that describe clinical oncology research protocols readable by most patients and their families? J Clin Oncol. 1994;12(10):2211–5. [DOI] [PubMed] [Google Scholar]
- 9. Cheung WY, Pond GR, Heslegrave RJ, Enright K, Potanina L, Siu LL. The contents and readability of informed consent forms for oncology clinical trials. Am J Clin Oncol. 2010;33(4):387–92. [DOI] [PubMed] [Google Scholar]
- 10. Schumacher A, Sikov WM, Quesenberry MI, et al. Informed consent in oncology clinical trials: a Brown University Oncology Research Group prospective cross-sectional pilot study. PLoS One. 2017;12(2):e0172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. Informed consent for clinical trials: a comparative study of standard versus simplified forms. J Natl Cancer Inst. 1998;90(9):668–74. [DOI] [PubMed] [Google Scholar]
- 12. Kim EJ, Kim SH. Simplification improves understanding of informed consent information in clinical trials regardless of health literacy level. Clin Trials. 2015;12(3):232–6. [DOI] [PubMed] [Google Scholar]
- 13. Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J Clin Oncol. 2007;25(9):e13–e14. [DOI] [PubMed] [Google Scholar]
- 14. Reinert C, Kremmler L, Burock S, et al. Quantitative and qualitative analysis of study-related patient information sheets in randomised neuro-oncology phase III trials. Eur J Cancer. 2014;50(1):150–8. [DOI] [PubMed] [Google Scholar]
- 15. Berger O, Grønberg BH, Sand K, Kaasa S, Loge JH. The length of consent documents in oncological trials is doubled in twenty years. Ann Oncol. 2009;20(2):379–85. [DOI] [PubMed] [Google Scholar]
- 16. Koyfman SA, Agre P, Carlisle R, et al. Consent form heterogeneity in cancer trials: the cooperative group and institutional review board gap. J Natl Cancer Inst. 2013;105(13):947–53. [DOI] [PubMed] [Google Scholar]
- 17. Schaeffer MH, Krantz DS, Wichman A, Masur H, Reed E, Vinicky JK. The impact of disease severity on the informed consent process in clinical research. Am J Med. 1996;100(3):261–8. [DOI] [PubMed] [Google Scholar]
- 18. Bergenmar M, Johansson H, Wilking N. Levels of knowledge and perceived understanding among participants in cancer clinical trials—factors related to the informed consent procedure. Clin Trials. 2011;8(1):77–84. [DOI] [PubMed] [Google Scholar]
- 19. Campbell HM, Raisch DW, Sather MR, Segal AR, Warren SR, Naik R. Impact of a clinical trials information handbook on patient knowledge, perceptions, and likelihood of participation. IRB. 2008;30(1):6–14. [PubMed] [Google Scholar]
- 20. Maughan TS, Meade AM, Adams RA, et al. A feasibility study testing four hypotheses with phase II outcomes in advanced colorectal cancer (MRC FOCUS3): a model for randomised controlled trials in the era of personalised medicine? Br J Cancer. 2014;110(9):2178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hutchison C, Cowan C, McMahon T, Paul J. A randomised controlled study of an audiovisual patient information intervention on informed consent and recruitment to cancer clinical trials. Br J Cancer. 2007;97(6):705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kraft SA, Constantine M, Magnus D, et al. A randomized study of multimedia informational aids for research on medical practices: implications for informed consent. Clin Trials. 2017;14(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tait AR, Voepel-Lewis T, Moscucci M, Brennan-Martinez CM, Levine R. Patient comprehension of an interactive, computer-based information program for cardiac catheterization: a comparison with standard information. Arch Intern Med. 2009;169(20):1907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rowbotham MC, Astin J, Greene K, Cummings SR. Interactive informed consent: randomized comparison with paper consents. PLoS One. 2013;8(3):e58603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kass NE, Sugarman J, Medley AM, et al. An intervention to improve cancer patients’ understanding of early-phase clinical trials. IRB. 2009;31(3):1–10. [PMC free article] [PubMed] [Google Scholar]
- 26. Fink AS, Prochazka AV, Henderson WG, et al. Enhancement of surgical informed consent by addition of repeat back: a multicenter, randomized controlled clinical trial. Ann Surg. 2010;252(1):27–36. [DOI] [PubMed] [Google Scholar]
- 27. Festinger DS, Marlowe DB, Croft JR, Dugosh KL, Arabia PL, Benasutti KM. Monetary incentives improve recall of research consent information: it pays to remember. Exp Clin Psychopharmacol. 2009;17(2):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown RF, Butow PN, Boyle F, Tattersall MH. Seeking informed consent to cancer clinical trials; evaluating the efficacy of doctor communication skills training. Psychooncology. 2007;16(6):507–16. [DOI] [PubMed] [Google Scholar]
- 29. Donovan J, Mills N, Smith M, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ. 2002;325(7367):766–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazanec S, Daly B, Meropol NJ, Step M. Facilitating enrollment in a Cancer registry through modified consent procedures: a pilot study. J Empir Res Hum Res Ethics. 2012;7(5):71–5. [DOI] [PubMed] [Google Scholar]
- 31. de Salis I, Tomlin Z, Toerien M, Donovan J. Qualitative research to improve RCT recruitment: issues arising in establishing research collaborations. Contemp Clin Trials. 2008;29(5):663–70. [DOI] [PubMed] [Google Scholar]
- 32. Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy SA. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Stat Med. 2012;31(17):1887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Belani CP, Barstis J, Perry MC, et al. Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol. 2003;21(15):2933–9. [DOI] [PubMed] [Google Scholar]
- 34. Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27(7):1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thall PF, Logothetis C, Pagliaro LC, et al. Adaptive therapy for androgen-independent prostate cancer: a randomized selection trial of four regimens. J Natl Cancer Inst. 2007;99(21):1613–22. [DOI] [PubMed] [Google Scholar]
- 36. Mateos MV, Oriol A, Martinez-Lopez J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11(10):934–41. [DOI] [PubMed] [Google Scholar]
- 37. Kuruvilla J, Kouroukis CT, Benger A, et al. A randomized trial of rituximab vs observation following autologous stem cell transplantation (ASCT) for relapsed or refractory CD20-positive B cell lymphoma: final results of NCIC CTG LY.12. Blood. 2013;122(21):155.23847185 [Google Scholar]
- 38. Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490–96. [DOI] [PubMed] [Google Scholar]
- 39. Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–7. [DOI] [PubMed] [Google Scholar]
- 40. van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108(10):3295–301. [DOI] [PubMed] [Google Scholar]
- 41. Stone RM, Berg DT, George SL, et al. Postremission therapy in older patients with de novo acute myeloid leukemia: a randomized trial comparing mitoxantrone and intermediate-dose cytarabine with standard-dose cytarabine. Blood. 2001;98(3):548–53. [DOI] [PubMed] [Google Scholar]
- 42. Matutes E, Bosanquet AG, Wade R, Richards SM, Else M, Catovsky D. The use of individualized tumor response testing in treatment selection: second randomization results from the LRF CLL4 trial and the predictive value of the test at trial entry. Leukemia. 2013;27(2):507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–53. [DOI] [PubMed] [Google Scholar]
- 44. Tournigand C, Chibaudel B, Samson B, et al. Improving safety in first-line metastatic colorectal cancer (MCRC) therapy with bevacizumab: modified FOLFOX7 versus XELOX2—results of the induction phase of the GERCOR DREAM randomized phase III study. J Clin Oncol. 2015;33(3 Suppl.):670.25605836 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Online_Supplement_online_supp for The Challenges of Informed Consent in High-Stakes, Randomized Oncology Trials: A Systematic Review by Julia M. Nathe and Elizabeth F. Krakow in MDM Policy & Practice