Abstract
Background:
Systemic lupus erythematosus (SLE) is a complex autoimmune disease associated with an elevated risk for premature cardiovascular disease. Platelets express receptors contributing to inflammation and immunity including FcγRIIA, the low affinity receptor of the Fc portion of IgG antibodies. The variation at a single amino acid substitution, H131R, in the extracellular binding domain alters the affinity for IgG, which may account for individual variation in platelet activity and platelet mediated disease.
Objectives:
This study was performed to investigate the association between FcγRIIA genotype, preclinical atherosclerosis, platelet reactivity, and vascular health.
Methods:
FcγRIIA was genotyped in 80 SLE patients and 30 healthy controls. Carotid ultrasound plaque, soluble E-selectin, and platelet aggregability were evaluated in SLE and matched controls.
Results:
Carotid plaque was significantly more prevalent in SLE patients carrying a variant allele compared to those who were homozygous ancestral (58% vs. 25%, P=0.04). In contrast, prevalent carotid plaque was not associated with genotype in controls. Consistently, SLE variant FcγRIIA carriers vs. ancestral had a significant increase in the levels of soluble E-selectin, which was not observed in controls. Monocyte and leukocyte-platelet aggregation and platelet aggregation in response to submaximal agonist stimulation were significantly elevated in SLE patients with the variant vs. ancestral genotype.
Conclusions:
Carotid ultrasound plaque, soluble E-selectin levels and platelet activity were more frequently prevalent in SLE patients carrying variant FcγRIIA. The interplay between FcγRIIA-mediated platelet activation and endothelial cells might represent a mechanism underlying the pathogenesis of cardiovascular disease in SLE patients.
Keywords: Atherosclerosis, cardiovascular disease, FcγRIIA, platelets, systemic Lupus Erythematosus (SLE)
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with an elevated risk for premature cardiovascular disease (CVD). A growing body of evidence supports the notion that CVD is the cause of death in the majority of patients with longstanding SLE [1–3]. Indeed, the rate of myocardial infarction in women with SLE aged 35–44 years is ~50 times greater than expected [1]. Subclinical CVD, including intima-media thickness (IMT), carotid plaque and coronary artery calcium is significantly higher in SLE than controls [4, 5]. Increased atherosclerotic risk in SLE remains despite adjustment for traditional Framingham risk factors [2]. Additional risk factors may include longer duration of disease and lower likelihood of treatment with prednisone, cyclophosphamide, or hydroxychloroquine [4], a combination that may promote persistent inflammation leading to vascular tissue damage and induction of CVD.
Among the expanding list of nontraditional biomarkers, platelets have been understudied as a relevant contributor to premature atherosclerosis in SLE. Pathological and clinical studies consistently demonstrate that platelets play a key role in atherosclerosis and thrombosis. Platelets, which contain transcripts and the necessary molecular machinery to conduct translation, are intercellular regulators of inflammation and immune activation. Increasing evidence suggests the contribution of platelets to the pathogenesis of SLE. As such, platelets isolated from subjects with SLE compared to controls reveal evidence of hyperactivity as supported by increased membrane expression of P-selectin and CD40 ligand [6, 7], higher binding of Annexin V [7, 8], complement deposition [9, 10] and platelet-leukocyte complexes [6, 8, 11]. In a lupus murine model, depletion of platelets or administration of a potent antiplatelet drug, clopidogrel, improved various measures of disease severity and overall survival [6]. A recent study by our group demonstrated that platelets are hyperreactive in SLE and can contribute to the pathogenesis of vascular disease in these patients by activating endothelial cells in an IL1-β dependent manner [12].
Platelets express immune receptors such the activating Fcγ receptor IIA (FcγRIIA), which binds with low affinity to the Fc portion of immunoglobulin G (IgG) [13]. IgG binding to cognate antigens form immune complexes (ICs) that have been shown to activate platelets. Recent studies of influenza and heparin induced thrombocytopenia, have identified a hyperactive platelet phenotype in subjects with persistent and elevated circulating ICs and a putative role of FcγRIIA signaling. FcγRIIA harbors a functional polymorphism due to a single amino acid substitution, H131R, in the extracellular binding domain resulting in altered affinity to IgG2 [14]. Functional variation in this family of receptors has been associated with SLE and lupus nephritis across diverse populations [15, 16].
Based on the plausible contribution of platelet activity to atherosclerotic risk in SLE, this study was initiated to determine the functional consequences of variation at FcγRIIA in SLE platelets. The approach leveraged a previously reported cohort of SLE patients in whom carotid IMT with inflammatory biomarkers and banked DNA was available for FcγRIIA genotyping [4, 5]. In addition, a newly assembled cohort was genotyped and platelet activity phenotyped to assess functional associations.
Materials and Methods
Study Population
Patients were recruited from the NYU Langone Medical Center and Bellevue Hospital. Written informed consent approved by the NYU institutional review board (IRB) was obtained from all subjects. All patients fulfilled at least 4 of the American College of Rheumatology (ACR) Criteria for the diagnosis of SLE [17]. The first cohort was previously published [5] and comprised patients with SLE and healthy controls evaluated by carotid ultrasound to determine the prevalence of atherosclerosis and associated inflammatory markers. This group was available for the determination of rs1801274 genotypes in FcγRIIA. The second cohort included patients enrolled based on consecutive attendance in either of two specialized lupus clinics or Rheumatology private practices as well as healthy controls. These patients and controls contributed fresh platelets for functional evaluations and genotyping. Exclusion criteria in this second cohort included the use of over-the-counter or prescribed non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, or any anticoagulation, thrombocytopenia or anemia.
Phlebotomy and Sample Processing
Blood was collected using a 21-gauge needle. Following a 2cc discard, blood was collected into vacutainer tubes containing EDTA for complete blood count (CBC), 3.2% (0.105 moles/L) sodium citrate for platelet collections, platelet activity measures, and plasma aliquots (Becton Dickenson, Franklin Lakes, NJ). Serum separator tubes were used to collect serum (SST; Becton Dickinson). After phlebotomy, blood was immediately transported to the laboratory for processing. Sodium citrate anti-coagulated blood was centrifuged within 15 minutes of phlebotomy at 200g for 10 minutes, yielding platelet rich plasma (PRP) that was used for light transmission aggregometry. To obtain serum, blood in SST tubes was allowed to clot for 30 minutes at room temperature and then centrifuged at 2500g for 10 minutes. Plasma and serum were aliquoted immediately after completion of centrifugation, flash frozen in liquid nitrogen, and stored at −80°C until the time of assay. From a purified DNA fraction, genotyping of FcγRIIA was performed by the allelic exclusion technique using assays and reagents purchased from Applied Biosciences (SNP ID: rs1801274, Assay ID: C___9077561_20), and assignments were confirmed by direct sequencing.
Measurement of soluble E-Selectin
As described previously [5], sE-selectin levels were measured using an enzyme-linked immunosorbent assay (ELISA) according to the instructions of the manufacturers (R&D Biosystems).
Light Transmission Aggregometry
Light transmission aggregation (LTA) was performed using AggRAM light transmission aggregometer, reagents, cuvettes, and stir bars (Helena Laboratories, Beaumont, Texas). Agonists were purchased from a single lot at the onset of the study. LTA was performed according to manufacturer’s specification and tests ran for 10 minutes. As described previously [18], citrate-anticoagulated blood was centrifuged at 200g for 10 minutes to obtain platelet rich plasma (PRP). LTA was immediately performed, and platelet aggregation was assessed in response to submaximal epinephrine, collagen, adenosine diphosphate (ADP), and arachidonic acid (AA), in that respective order. Testing of the latter agonists was dependent on availability of PRP, as the amount of PRP isolated from each subject varied.
Flow Cytometry
To assess Leukocyte-Platelet Aggregates (LPA) and Monocyte-Platelet Aggregates (MPA), citrate anti-coagulated blood was fixed with 1% formaldehyde in a 1:1.2 ratio and stained with fluorescently conjugated antibodies; CD61-FITC (platelets) and either CD14-APC (monocytes) or CD45-APC (leukocytes) for 10 minutes in the dark at room temperature. Monocytes and leukocytes were collected based on side-scatter properties and positive staining for CD14 or CD45, respectively. Monocyte-platelet aggregates were identified as having a positive stain for CD14 and CD61, and LPA were identified as having a positive stain for CD45 and CD61. Data are expressed as a percentage of at least 25,000 leukocytes or 2,000 monocytes positive for adherent platelets. Data were acquired on BD Accuri C6 Cytometer (BD Biosciences) and were analyzed using FlowJo (Tree Star) software.
Statistical Analysis
The Student unpaired t-test was used in 2-group comparisons of normally distributed data, whereas the Mann-Whitney nonparametric test was used when the normality assumption was not met. Fisher’s exact test was performed to evaluate bivariate associations between categorical variables. Statistical significance was performed using GraphPad Prism version 6.0g (GraphPad Software, La Jolla, California, USA). All P values were considered significant at P ˂ 0.05.
Results and discussion
The demographic and clinical profile of the SLE patients and controls are shown in Table 1 (Page 17). Subjects were mostly female and nearly 50% white. Controls were matched by age, sex, and race/ethnicity. Approximately two-thirds of the population fulfilled the renal ACR classification criteria for SLE. As reported previously, SLE patients had a significantly higher prevalence of carotid intima thickness (CIT).
Table 1.
Demographics of Patients and Controls
| Cohort | SLE-1 | Control-1 | SLE-2 |
|---|---|---|---|
| Evaluation | Carotid IMT | Carotid IMT | Platelet activity |
| N | 49 | 30 | 31 |
| Age, mean | 45.0 ± 7.5 | 42.6 ± 8.3 | 40 ± 12.4 |
| Female, n (%) | 44 (95.6) | 25 (86.2) | 20 (83..3) |
| White, % | 43.4 | 51.7 | 46.6 |
| Malar rash*, % | 63.1 | 53.3 | |
| Discoid rash*, % | 36.8 | 50 | |
| Photosensitivity*, % | 56.7 | 50 | |
| Oral ulcers*, % | 47.3 | 36.6 | |
| Arthritis*, % | 94.8 | 73.3 | |
| Serositis*, % | 35.1 | 33 | |
| Renal*, % | 58.3 | 73.3 | |
| Neurological, % | 5.5 | 0 | |
| rs1801274 MAF | 0.41 | 0.41 | 0.38 |
ACR criteria
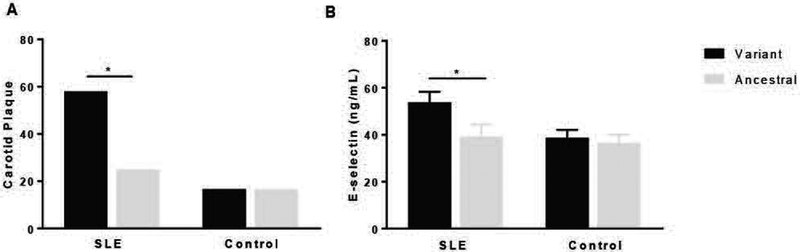
Overall, allelic frequencies of FcγRIIA were not significantly different between SLE patients or healthy controls. Minor allele frequency (MAF) of rs1801274 was found in ≈40% of subjects with SLE and controls. There was no difference in demographics by FcγRIIA genotype. Among the 49 SLE subjects with carotid ultrasound assessment, 17 of 29 (58%) SLE patients with the allelic variant had carotid plaque compared to 5 of 20 (25%) SLE patients without the variant had carotid plaque (P=0.039, Figure 1A). In contrast, the presence of the FcγRIIA rs1801274 variant did not associate with carotid plaque in healthy controls (Figure 1A).
Figure 1.
Increased prevalence of atherosclerotic plaque and E-selectin levels in systemic lupus erythematosus (SLE) with FcγRIIA variant. (A) Atherosclerotic plaque was measured using ultrasonography in SLE patients (n= 49) and controls (n= 30). Increased prevalence of plaque was observed in SLE patients with variant FcγRIIA compared to SLE patients with ancestral FcγRIIA. In contrast, there was no significant difference in plaque prevalence in controls with or without the variant FcγRIIA. (B) Soluble E-selectin (sE-selectin) was measured in serum of patients with SLE (n= 34) and healthy controls (n= 42) carrying either the variant or ancestral form of FcγRIIA. P-value was calculated using Unpaired t-Test (*, p < 0.05).
There are biologic consequences of allelic variation in the Fc receptors. These include differences in immune complex clearance by phagocytic cells as well as downstream signaling pathways [15, 16]. Zhou and coworkers demonstrated that FcγRIIA receptor signaling in platelets induced by FcγRIIA triggering, directly contributed to a hyperreactive phenotype and was dependent on the H131R polymorphism. Specifically, platelets isolated from R/R131 homozygotes (variant) were highly responsive and H/H 131 homozygotes (ancestral) platelets were weakly responsive to stimulation via FcγRIIA [19]. In support of this concept, Chen and coworkers demonstrated that the H131R polymorphism of FcγRIIA is important for fibrinogen binding to the αIIbβ3 [20]. In addition to platelets, FcγRIIA is also expressed by other immune cells and as such could potentially contribute to the development of atherosclerosis.
We previously showed that patients with SLE have elevated soluble E-selectin, and among SLE patients, soluble E-selectin was higher in those with (versus without) CIT [5]. In this cohort, the levels of soluble E-selectin were higher in SLE subjects carrying a variant allele compared to those with ancestral alleles (P=0.036, Figure 1B). In contrast, soluble E-selectin did not differ by allelic frequencies of FcγRIIA rs1801274 among healthy controls. A non-specific marker of inflammation, C-reactive protein, did not differ significantly among SLE patients or controls carrying the variant allele compared to those with the homozygous ancestral genotype (data not shown).
SLE is characterized by an abundance of ICs, which contribute to systemic and tissue inflammation. Platelets which are in close interaction with endothelial cells of the vascular wall, can promote CVD by functioning as a bridging link between ICs and endothelial cells. By cross linking the variant FcγRIIA, IgG-containing ICs can increase platelet aggregation, which in turn enhances the activation of endothelial cells. Indeed, we have recently demonstrated that platelets induce endothelial inflammation in an IL-1β dependent manner [12]. Elevation of sE-selectin in subjects carrying the variant FcγRIIA, is consistent with the notion that this biomarker reflects activation of the endothelium and high levels have been previously associated with atherosclerosis and cardiovascular risk in both SLE and non SLE cohorts [5, 21–23]
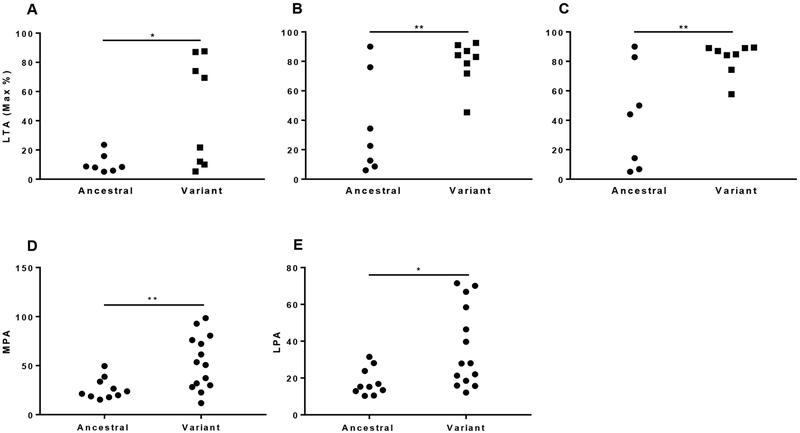
Given prior data from our group demonstrating that platelets from SLE patients exhibit an activated phenotype and an ability to induce proinflammatory endothelial cell phenotype [12], platelet aggregation and platelet-leukocyte interaction were explored. Since platelet aggregation in response to submaximal epinephrine stimulation is a robust marker of platelet activity that generalizes to a global hyperreactive platelet phenotype, we investigated platelet aggregation in response to low-epinephrine. Patients with SLE and the allelic variant had increased aggregation in response to epinephrine 0.1 μM, 0.4 μM and 2 μM compared to SLE patients with the homozygous ancestral genotype (P<0.05 for each assessment; (Figure 2A, B and C). Consistently, SLE patients with the allelic variant had a higher percent aggregation to low-dose (160 μM) arachidonic acid (data not shown). While numerically higher, no significant difference was detected in platelet aggregation in response to low-dose collagen (0.2 μg/ml) or ADP (0.4 μM) (data not shown). Cross-talk between platelets and leukocytes is a crucial pathophysiological mechanism linking atherothrombosis, immunity and inflammation, and ex vivo measurement of monocyte- (and leukocyte) platelet aggregation in the circulation has been proposed to represent a robust biomarker of platelet activation in vivo. Both MPA and LPA were significantly higher in SLE patients carrying the variant allele compared to those with the homozygous ancestral genotype (Figure 2D and E).
Figure 2.
Increased platelets activity in SLE patients with the variant FcγRIIA. Light transmission aggregation (LTA) was measured following treatment of platelet-rich plasma of SLE patients with 0.1 μM (A), 0.4 μM (B) or 2 μM (C) epinephrine by using Helena AggRAM light transmission aggregometer. The percentage of monocyte-platelet aggregates (MPA, D)) and leukocyte-platelet aggregates, (LPA, E) assessed by flow cytometry. Single dots in the graphs represent measurement of individual patients. Statistical significance was determined using Unpaired t-Test (*, p < 0.05; **, p < 0.01).
Several limitations exist when interpreting the results of our study. First, the findings include two separate cohorts and individuals with IMT and measurement of E-selectin, did not have evaluations of platelet function (and vice versa). Second, atherosclerosis develops over years and our data on platelet and vascular functional parameters were limited to cross sectional analyses. Finally, our population was not large enough to look at heterozygous carriers (R/H) of the variant form of FcγRIIA.
Altogether, the data of this study supports the contributing role of FcyRIIA genotype in premature atherosclerosis, vascular impairment and increased platelet activity in patients with SLE.
Essentials.
Systemic lupus erythematosus (SLE) patients are at increased risk for premature CVD.
Platelet activity, vascular dysfunction and carotid artery plaque are associated with FcγRIIA genotype in SLE.
FcγRIIA genotype was not associated with platelet activity or carotid plaque in healthy controls.
FcγRIIA represents a link that connects platelet activity, vascular health and CVD in SLE.
Acknowledgements
This work was supported by a National Institutes of Health grant R21 AR071103–01 (JPB, JSB).
Footnotes
Disclosure of conflict of interests
H. Reynolds reports non-financial support from Abbott Vascular, outside the submitted work. The other authors state that they have no conflict of interest.
References
- 1.Urowitz MB, Gladman D, Ibanez D, Fortin P, Sanchez-Guerrero J, Bae S, Clarke A, Bernatsky S, Gordon C, Hanly J, Wallace D, Isenberg D, Ginzler E, Merrill J, Alarcon G, Steinsson K, Petri M, Dooley MA, Bruce I, Manzi S, et al. Clinical manifestations and coronary artery disease risk factors at diagnosis of systemic lupus erythematosus: data from an international inception cohort. Lupus 2007;16:731–5. [DOI] [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr., Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. American journal of epidemiology 1997;145:408–15. [DOI] [PubMed] [Google Scholar]
- 3.Giannelou M, Mavragani CP. Cardiovascular disease in systemic lupus erythematosus: A comprehensive update. J Autoimmun 2017;82:1–12. [DOI] [PubMed] [Google Scholar]
- 4.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, Crow MK, Schwartz JE, Paget SA, Devereux RB, Salmon JE. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. The New England journal of medicine 2003;349:2399–406. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds HR, Buyon J, Kim M, Rivera TL, Izmirly P, Tunick P, Clancy RM. Association of plasma soluble E-selectin and adiponectin with carotid plaque in patients with systemic lupus erythematosus. Atherosclerosis 2010;210:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I, Bordes C, Viallard JF, Goulvestre C, Pellegrin JL, Weil B, Moreau JF, Batteux F, Blanco P. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med 2010;2:47ra63. [DOI] [PubMed] [Google Scholar]
- 7.Nagahama M, Nomura S, Ozaki Y, Yoshimura C, Kagawa H, Fukuhara S. Platelet activation markers and soluble adhesion molecules in patients with systemic lupus erythematosus. Autoimmunity 2001;33:85–94. [DOI] [PubMed] [Google Scholar]
- 8.Lood C, Amisten S, Gullstrand B, Jonsen A, Allhorn M, Truedsson L, Sturfelt G, Erlinge D, Bengtsson AA. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood 2010;116:1951–7. [DOI] [PubMed] [Google Scholar]
- 9.Lood C, Tyden H, Gullstrand B, Sturfelt G, Jonsen A, Truedsson L, Bengtsson AA. Platelet activation and anti-phospholipid antibodies collaborate in the activation of the complement system on platelets in systemic lupus erythematosus. PLoS One 2014;9:e99386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lood C, Eriksson S, Gullstrand B, Jonsen A, Sturfelt G, Truedsson L, Bengtsson AA. Increased C1q, C4 and C3 deposition on platelets in patients with systemic lupus erythematosus--a possible link to venous thrombosis? Lupus 2012;21:1423–32. [DOI] [PubMed] [Google Scholar]
- 11.Joseph JE, Harrison P, Mackie IJ, Isenberg DA, Machin SJ. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br J Haematol 2001;115:451–9. [DOI] [PubMed] [Google Scholar]
- 12.Nhek S, Clancy R, Lee KA, Allen NM, Barrett TJ, Marcantoni E, Nwaukoni J, Rasmussen S, Rubin M, Newman JD, Buyon JP, Berger JS. Activated Platelets Induce Endothelial Cell Activation via an Interleukin-1beta Pathway in Systemic Lupus Erythematosus. Arterioscler Thromb Vasc Biol 2017;37:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maenaka K, van der Merwe PA, Stuart DI, Jones EY, Sondermann P. The human low affinity Fcgamma receptors IIa, IIb, and III bind IgG with fast kinetics and distinct thermodynamic properties. J Biol Chem 2001;276:44898–904. [DOI] [PubMed] [Google Scholar]
- 14.Arepally G, McKenzie SE, Jiang XM, Poncz M, Cines DB. Fc gamma RIIA H/R 131 polymorphism, subclass-specific IgG anti-heparin/platelet factor 4 antibodies and clinical course in patients with heparin-induced thrombocytopenia and thrombosis. Blood 1997;89:370–5. [PubMed] [Google Scholar]
- 15.Manger K, Repp R, Spriewald BM, Rascu A, Geiger A, Wassmuth R, Westerdaal NA, Wentz B, Manger B, Kalden JR, van de Winkel JG. Fcgamma receptor IIa polymorphism in Caucasian patients with systemic lupus erythematosus: association with clinical symptoms. Arthritis and rheumatism 1998;41:1181–9. [DOI] [PubMed] [Google Scholar]
- 16.Brown EE, Edberg JC, Kimberly RP. Fc receptor genes and the systemic lupus erythematosus diathesis. Autoimmunity 2007;40:567–81. [DOI] [PubMed] [Google Scholar]
- 17.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 18.Merolla M, Nardi MA, Berger JS. Centrifugation speed affects light transmission aggregometry. Int J Lab Hematol 2012;34:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Abraham S, Andre P, Edelstein LC, Shaw CA, Dangelmaier CA, Tsygankov AY, Kunapuli SP, Bray PF, McKenzie SE. Anti-miR-148a regulates platelet FcgammaRIIA signaling and decreases thrombosis in vivo in mice. Blood 2015;126:2871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Dong JF, Sun C, Bergeron A, McBride L, Pillai M, Barnard MR, Salmon J, Michelson AD, Bray PF. Platelet FcgammaRIIA His131Arg polymorphism and platelet function: antibodies to platelet-bound fibrinogen induce platelet activation. J Thromb Haemost 2003;1:355–62. [DOI] [PubMed] [Google Scholar]
- 21.Rho YH, Chung CP, Oeser A, Solus J, Raggi P, Gebretsadik T, Shintani A, Stein CM. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumatol 2008;35:1789–94. [PMC free article] [PubMed] [Google Scholar]
- 22.Rohde LE, Lee RT, Rivero J, Jamacochian M, Arroyo LH, Briggs W, Rifai N, Libby P, Creager MA, Ridker PM. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arterioscler Thromb Vasc Biol 1998;18:1765–70. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM Jr., Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997;96:4219–25. [DOI] [PubMed] [Google Scholar]