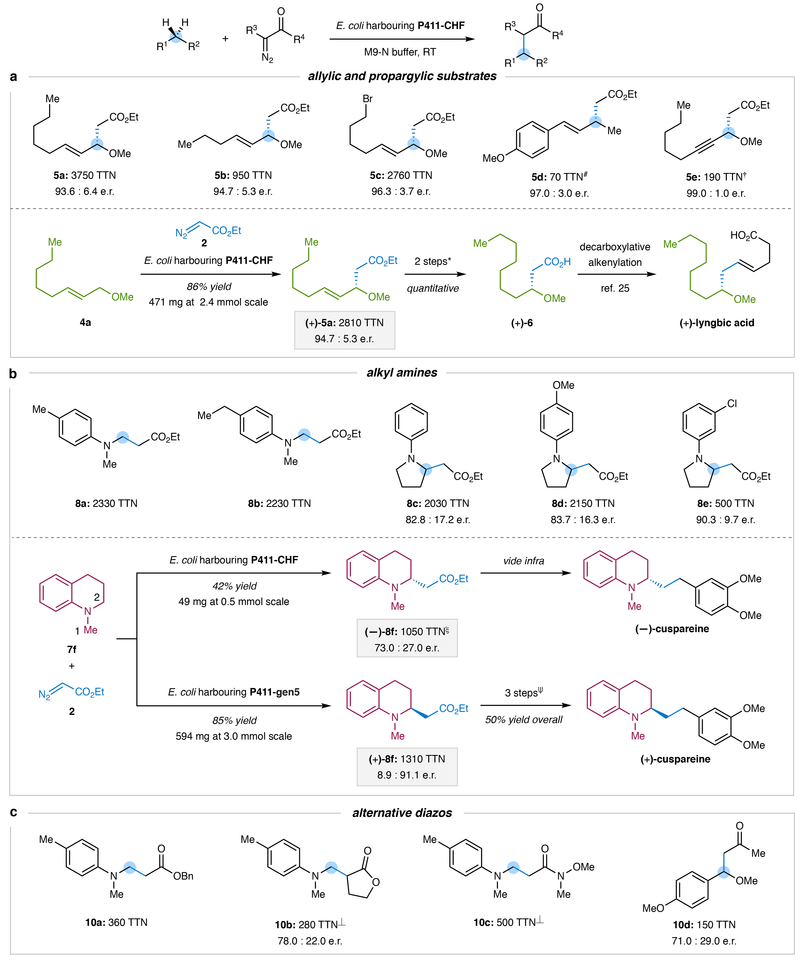
Figure 4 |. Application of P411 enzymes for sp3 C–H alkylation.
a, Allylic and propargylic C–H alkylation. Unless otherwise indicated, experiments were performed using E. coli expressing cytochrome P411-CHF with 10 mM substrate 4a–4e and 10 mM ethyl diazoacetate; each reported TTN is the average of quadruplicate reactions. #TTN was calculated based on isolated yield from a reaction performed at 0.25 mmol scale. †Cyclopropene product was also observed (Supplementary Fig. S8). *Hydrogenation, followed by hydrolysis. b, Enzymatic alkylation of substrates containing α-amino C–H bonds. Unless otherwise indicated, experiments were performed at 0.5 mmol scale using E. coli expressing cytochrome P411-CHF with substrates 7a–7f and ethyl diazoacetate; TTNs were calculated based on isolated yields of products shown. ξIsolated in 9 : 1 r.r. for 8f : 8f’. eReduction, halogen exchange, and Suzuki-Miyaura cross-coupling. c, Enzymatic C–H alkylation with alternative diazo reagents. Unless otherwise indicated, reactions were performed at 0.5 mmol scale using E. coli expressing cytochrome P411-CHF with coupling partner 1a or 7a and diazo compounds 9a–9d; TTNs were calculated based on isolated yields of products shown. ⏊Variant P411-IY T327I was used. See Supplementary Information for the complete list substrates (Fig. S12 and Fig. S13), information about enzyme variants, and full experimental details.