Abstract
Although visceral artery aneurysms are rare, mortality due to their rupture is high, estimated at even 25–75%. That is why it is significant to detect each such lesion. Visceral artery aneurysms are usually asymptomatic and found incidentally during examinations performed for other indications. Autopsy results suggest that most asymptomatic aneurysms remain undiagnosed during lifetime. Their prevalence in the population is therefore higher. The manifestation of a ruptured aneurysm depends on its location and may involve intraperitoneal hemorrhage, gastrointestinal and portal system bleeding with concomitant portal hypertension and bleeding from esophageal varices. Wide access to diagnostic tests, for example ultrasound, computed tomography or magnetic resonance imaging, helps establish the correct diagnosis and a therapeutic plan as well as select appropriate treatment. After a procedure, the same diagnostic tools enable assessment of treatment efficacy, or are used for the monitoring of aneurysm size and detection of potential complications in cases that are ineligible for treatment. The type of treatment depends on the size of an aneurysm, the course of the disease, risk of rupture and risk associated with surgery or endovascular procedure. Endovascular treatment is preferred in most cases. Aneurysms are excluded from the circulation using embolization coils, ethylene vinyl alcohol, stents, multilayer stents, stent grafts and histoacryl glue (or a combination of these methods).
Keywords: aneurysm, arteries, Doppler ultrasound
Introduction
Visceral artery aneurysms are rare. Post-mortem examinations have demonstrated their presence in 0.1–1.4% of cases(1,2). Mortality due to their rupture is high, estimated at even 25–75%(1,2). That is why it is significant to detect each such lesion. At present, visceral artery aneurysms are being identified more and more frequently thanks to the development of imaging techniques(3).
Visceral artery aneurysms are usually asymptomatic and found incidentally during examinations performed for other indications. The manifestation of a ruptured aneurysm depends on its location and may involve intraperitoneal hemorrhage, gastrointestinal and portal system bleeding with concomitant portal hypertension and bleeding from esophageal varices(1).
Out of all visceral artery aneurysms, 60% are found in the splenic artery, 20% in the hepatic artery, 5.5% in the superior mesenteric artery, 4% in the celiac artery, 2% in the pancreatic branches, 1.5% in the gastroduodenal artery(4,5), and 1% in the inferior mesenteric artery.
After the abdominal aorta and iliac arteries, the splenic artery is the third most common location of abdominal aneurysms(4,6). These lesions are small, saccular and usually identified in the central or distal segment of the splenic artery (Fig. 1, Fig. 2)(5). Over 95% of these abnormalities are asymptomatic(6). Portal hypertension, fibro-muscular dysplasia, pancreatitis, atherosclerosis, abdominal trauma, vascular inflammatory diseases and septic emboli are the most frequent causes of splenic artery aneurysms(1). These lesions are 4 times more common in females(4,5,7), which is related with multiparity and pregnancy(4). The risk of rupture is estimated at 2–3%(4) and increases in patients after liver transplantation, during pregnancy and with portal hypertension(4,6). Mortality after rupture is 10–25%, while rupture in pregnant women results in maternal death in even 70% of cases and fetal death in over 90% of cases(7).
Fig. 1.
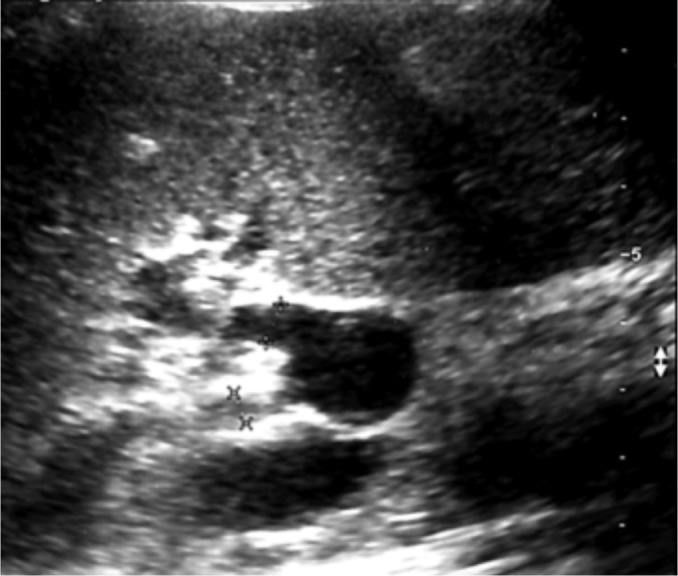
Splenic artery aneurysm in the splenic hilum – B-mode
Fig. 2.
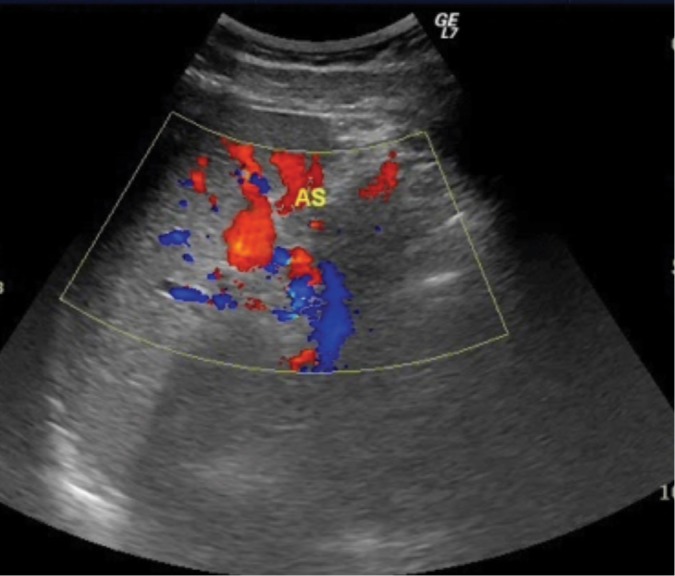
Splenic artery aneurysm in the splenic hilum – color-coded blood flow
Hepatic artery aneurysms are more common in males(1,4,5,8), and the prime causes of their development are atherosclerosis, mediointimal degeneration, trauma, infection and iatrogenic factors(9). These lesions are typically extra-hepatic (63%), located in the proper and common hepatic artery(5). Patients usually report no symptoms. Aneurysm rupture is associated with abdominal pain, gastrointestinal bleeding and the presence of blood in the bile ducts(4).
Superior mesenteric artery aneurysms tend to develop in the proximal segment of the vessel, usually along the first 5 cm(4). They are saccular or fusiform. Pseudoaneurysms are more common than true aneurysms(4). They are believed to develop secondary to infective endocarditis or infection associated with drug abuse(8). Patients are usually asymptomatic, and complications, if present, include mesenteric ischemia, thrombosis and rupture with massive hemorrhage(4). Mortality after aneurysm rupture is high, and reaches 30%(1).
Atherosclerosis, tunica media degeneration, trauma, vascular inflammatory diseases or infection are also the main causes of celiac artery aneurysms (Fig. 3)(1). These lesions are usually fusiform and located in the distal 1/3 of the vessel(8). Due to low risk of spontaneous rupture, conservative treatment and observation are recommended(1). Symptoms, such as abdominal pain, which may signal high likelihood of imminent rupture, are reported by 75% of patients(5).
Fig. 3.
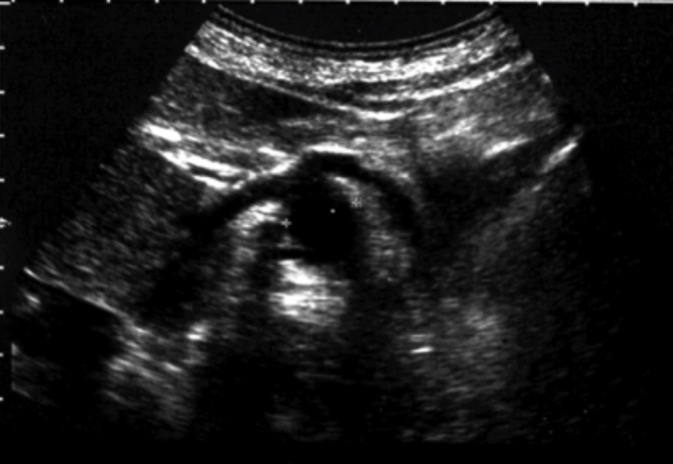
Celiac artery aneurysm – B-mode
Pancreaticoduodenal artery aneurysms are rare and mainly caused by pancreatitis or surgical interventions(1). They are usually pseudoaneurysms. Patients report abdominal pain; frequently a palpable mass is found in an abdominal examination(4). Each detected aneurysm in this location should be treated as the risk of rupture is related with its size(4).
Gastroduodenal artery aneurysms are the least common of all splanchnic aneurysms. They develop mainly in patients with pancreatitis, and most patients present signs and symptoms typical of pancreatitis(4).
Wide access to diagnostic tests, such as ultrasound, computed tomography or magnetic resonance imaging, helps establish the correct diagnosis and a therapeutic plan as well as select appropriate treatment. After a procedure, the same diagnostic tools enable assessment of treatment efficacy, or are used for the monitoring of aneurysm size and detection of potential complications in cases that are ineligible for treatment.
The type of treatment depends on the size of aneurysm, the course of the disease, risk of rupture and risk associated with surgery or endovascular procedure. Endovascular treatment is preferred in most cases. The main indications are: size greater than twice the caliber of the artery, rapidly increasing aneurysms, symptomatic aneurysms and aneurysms in women of child-bearing age due to high risk of rupture(8). Pseudoaneurysms require urgent treatment due to high risk of perforation(3). Most asymptomatic aneurysms need no treatment; only their observation is indicated.
Discussion
Visceral artery aneurysms are rare, accounting for 0.1–0.2% of all aneurysms(6). Autopsy results suggest that most asymptomatic aneurysms remain undiagnosed during lifetime. Their prevalence in the population is therefore higher(4).
Ultrasound is the first examination in patients with a suspected visceral aneurysm. These abnormalities are frequently detected incidentally during an ultrasound scan conducted for other indications. Ultrasound, which is readily available and non-invasive, is able to detect visceral artery aneurysms with similar accuracy to detecting abdominal aorta aneurysms(5). Typical signs are: dilated artery and thickened wall as well as the presence of atherosclerotic plaque or thrombus. Ultrasonography also enables morphological assessment of the wall and sac of aneurysms and hemodynamic evaluation of blood flow in their region. It is also useful for evaluation of concomitant atherosclerotic changes in the visceral arteries and for identifying patients with high risk of myocardial infarction or stroke by measuring the forearm–ankle index(1). In patients who are not well-prepared for examination, it is often impossible to visualize the retroperitoneal space and visceral vessels. Other factors hindering the diagnosis include obesity, calcified vascular wall and limited spatial resolution(5,10). Patients should be fasting for 6–8 hours and cannot drink for 2 hours before the test as food may significantly alter flow parameters in both the celiac and mesenteric arteries(11).
Computed tomography is currently the primary method for vascular imaging(4). It helps specify the location, shape and size of an aneurysm, assess the character of the wall, and determine the course and relationship to other vessels(6). This examination is mostly indicated in patients with severe abdominal trauma, or after recent interventional procedures within the biliary tree that might be complicated with aneurysms(1). Renal failure, no intravenous access and allergy to a contrast medium are contraindications to computed tomography, thus rendering diagnosis with this method impossible(10).
Magnetic resonance imaging has additional advantages compared to CT: contrast agents are not nephrotoxic and there is no ionizing radiation exposure(1). Magnetic resonance angiography is performed in patients with Marfan syndrome or vascular inflammatory diseases (Takayasu disease, giant cell arteritis) in whom the risk of an aneurysm is high(1). However, clips, stents and coils can cause artifacts which may render assessment difficult or even impossible(5).
Until recently, surgery was the preferred treatment of visceral artery aneurysms. It involved aneurysm resection with revascularization, aneurysm ligation, ligation of the artery with the aneurysm or resection of an organ with its supplying vessel(12,13). These procedures are characterized by high efficacy, while perioperative mortality does not exceed 5%(12). At present, endovascular procedures are the mainstay of treatment. Aneurysms are excluded from the circulation using embolization coils, EVOH, stents, multilayer stents, stent grafts and histoacryl glue or a combination of these methods(12). They are highly efficacious; Tulsyana et al. report treatment efficacy at the level of 98% based on 48 patients(14). Most authors believe that embolization with coils is the most effective treatment of visceral artery aneurysms(15). However, in the past several years, aneurysms have been repeatedly and effectively closed with covered stents(16–19). Also, combined methods are becoming more and more popular. These involve bare stents implanted on the segment with the aneurysm and embolization coils that are introduced through ports in the stent wall. The role of stents is to prevent the coil from protruding into the vascular lumen. This method is used in the treatment of broad-neck aneurysms(12,13). Moreover, bare multilayer stents are used to decrease blood flow into the aneurysmal sac, thereby excluding the aneurysm from the circulation(12). In aneurysms located in the distal segment of the vessel characterized by a tortuous course, thrombin embolization is an option(13).
Conclusion
Visceral artery aneurysms are believed to be rare, but their detectability has been increasing with the development of imaging modalities. Ultrasound is the first test conducted for diagnosing splanchnic aneurysms and monitoring asymptomatic lesions. Endovascular procedures are effective for both asymptomatic and ruptured visceral artery aneurysms.
Footnotes
Conflict of interest
Authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Badea R: Splanchnic artery aneurysms: The diagnostic contribution of ultrasonography in correlation with other imaging methods. J Gastrointestin Liver Dis 2008; 17: 101–105. [DOI] [PubMed] [Google Scholar]
- 2.Badea R, Barsan M, Scridon T, Miclaus G, Pascu O, Molnar G. et al. : Superior mesenteric artery aneurysm. importance of sonography as the primary imaging technique for detection. J Ultrasound Med 2010; 29: 1503–1506. [DOI] [PubMed] [Google Scholar]
- 3.Loffroy R, Favelier S, Pottecher P, Genson PY, Estivalet L, Gehin S. et al. : Endovascular management of visceral artery aneurysms: When to watch, when to intervene? World J Radiol 2015; 7: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horton KM, Smith C, Fishman EK: MDCT and 3D CT angiography of splanchnic artery aneurysms. Am J Roentgenol 2007; 189: 641–647. [DOI] [PubMed] [Google Scholar]
- 5.Pilleul F, Beuf O: Diagnosis of splanchnic artery aneurysms and pseudoaneurysms, with special reference to contrast enhanced 3D magnetic resonance angiography: A review. Acta Radiol 2004; 45: 702–708. [DOI] [PubMed] [Google Scholar]
- 6.Sułkowski L, Szura M, Pasternak A, Matyja M, Matyja A: Pathogenesis, diagnosis and treatment of splenic artery aneurysms. Austin J Vasc Med 2016; 3: 1017. [Google Scholar]
- 7.Jesinger R, Thoreson A, Lamba R: Abdominal and pelvic aneurysms and pseudyaneurysms: Imaging review with clinical, radiologic, and treatment correlation. Radiographics 2013; 33: 71–96. [DOI] [PubMed] [Google Scholar]
- 8.Jana M, Gamanagatti S, Mukund A, Paul S, Gupta P, Garg P. et al. : Endovascular management in abdominal visceral arterial aneurysms: A pictorial essay. World J Radiol 2011; 3: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara D, Giribomo AM, Viviani E, Padricelli A, Santagata A, Del Guercio L: Endovascular management of a large hepatic artery aneurysm. Clin Ter 2017; 168: 178–180. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal GA, Johnson PT, Fishman EK: Splenic artery aneurysms and pseudoaneurysms: Clinical disctinctions and CT appearances. Am J Roentgenol 2007; 188: 992–999. [DOI] [PubMed] [Google Scholar]
- 11.Elwertowski M, Lechowicz R: Standards of the Polish Ultrasound Society – update. Ultrasound examination of the visceral arteries. J Ultrason 2015; 15: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sojka M, Jargiełło T, Wolski A, Pyra K, Jarząbek M, Drelich-Zbroja A. et al. : Leczenie tętniaków trzewnych i nerkowych metodami wewnątrznaczyniowymi. Postępy Nauk Medycznych 2012; 5: 419–427. [Google Scholar]
- 13.Sojka M, Jargiełło T, Przyszlak M, Pyra K, Drelich-Zbroja A, Wolski A. et al. : Wewnątrznaczyniowe leczenie tętniaków tętnic trzewnych – doświadczenie jednego ośrodka. Acta Angiol 2011; 17: 209–218. [Google Scholar]
- 14.Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G. et al. : The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2007; 45: 276–283. [DOI] [PubMed] [Google Scholar]
- 15.Aranzulla TC, Colombo A, Sangiorgi GM: Succesful endovascular renal artery aneurysms exclusion using the Venture catheter and covered stent implantation: a case report and review of the literature. J Invasive Cardiol 2007; 19: 246–253. [PubMed] [Google Scholar]
- 16.Walton JM, Abraham RJ, Perey BJ, MacGregor JH, Campbell DR: Hepatic artery pseudoaneurysms in acute pancreatitits. Can J Surg 1991; 34: 377–380. [PubMed] [Google Scholar]
- 17.Gabelmann A, Görich J, Merkle EM: Endovascular treatment of visceral artery aneurysms. J Endovasc Ther 2002; 9: 38–47. [DOI] [PubMed] [Google Scholar]
- 18.Venturini M, Angeli E, Salvioni M, De Cobelli F, Trentin C, Carlucci M. et al. : Hemorrhage from a right hepatic artery pseudoaneurysm: Endovascular treatment with a coronary stent-graft. J Endovasc Ther 2002; 9: 221–224. [DOI] [PubMed] [Google Scholar]
- 19.Yoon HK, Lindh M, Uher P, Lindblad B, Ivancev K: Stent-graft repair of a splenic artery aneurysm. Cardiovasc Interv Radiol 2001; 24: 200–203. [DOI] [PubMed] [Google Scholar]


