Figure 1.
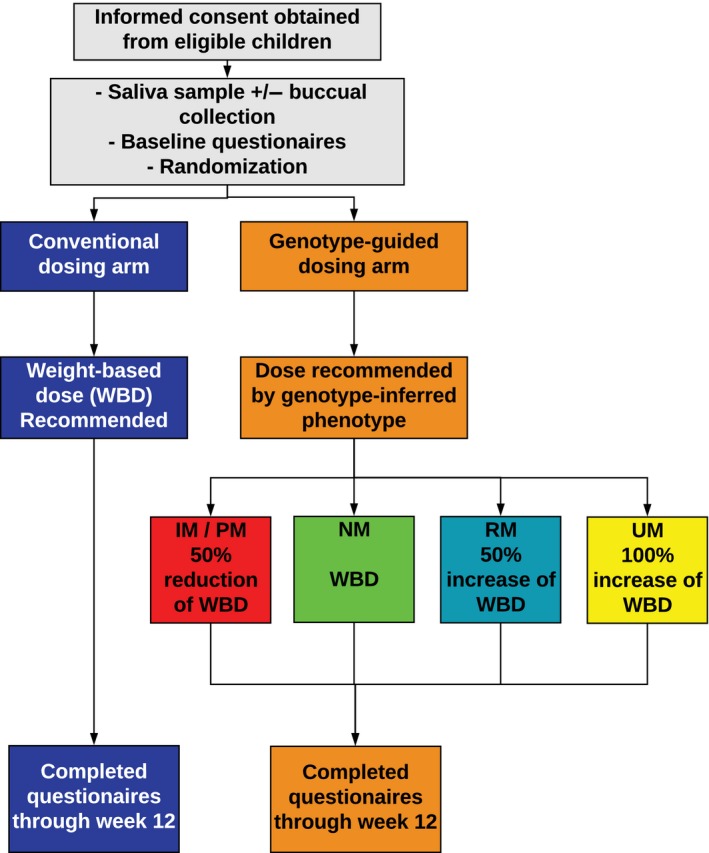
Study flow diagram. Children were eligible for the study if they were age 5‐17 years old and presented with symptoms of gastroesophageal reflux disease, or other gastric‐acid related conditions, where initiating a proton pump inhibitor (PPI) medication was warranted, or were on a subtherapeutic PPI. Once informed consent was obtained, participants provided a saliva sample and if enrolled from the Orlando site they also provided a buccal sample for genotyping, completed baseline questionnaires, and then were randomized 1:1 into either the conventional dosing arm or genotype‐guided dosing arm. Prescribers received a recommendation for the participant's PPI dose, once the participant started the PPI they completed questionnaires on a weekly basis throughout the study. IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer; RM, rapid metabolizer; UM, ultrarapid metabolizer.
