Figure 3.
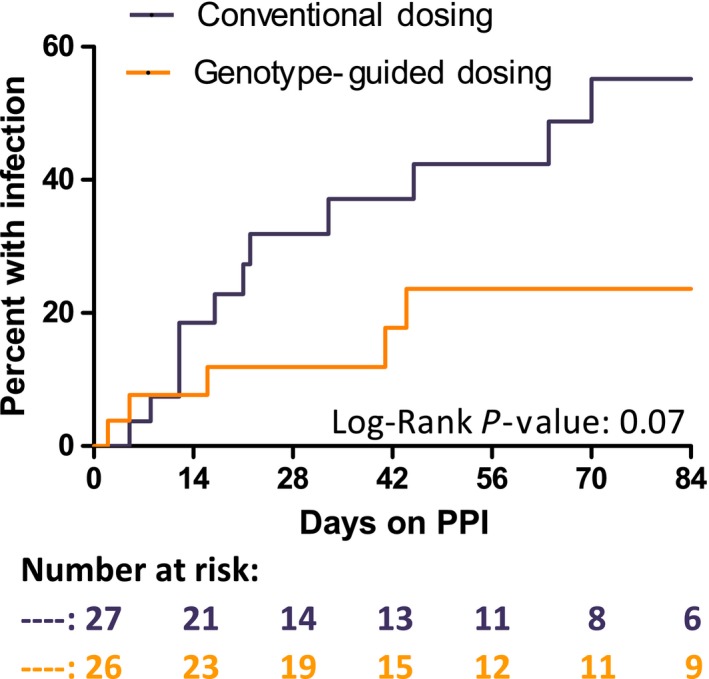
Kaplan‐Meier curve for infections reported in the Safety Questionnaire. Occurrence of upper respiratory infection, sore throat, or acute sinusitis over the 12‐week period or last date of follow‐up with participant was included (total n = 53). Participants who did not start a proton pump inhibitor (PPI; n = 5) or who reported an infection on the same day as they started PPI therapy (n = 2) were excluded from analysis. The conventional dosing (CD) arm included 27 participants with 12 events and the genotype‐guided dosing (GGD) arm included 26 participants with 5 events. The mean infection‐free time was 70 days for the CD arm and undefined for the GGD arm. The unadjusted hazard ratio was 2.42 with a 95% confidence interval of 0.93–6.29.
