Figure 2.
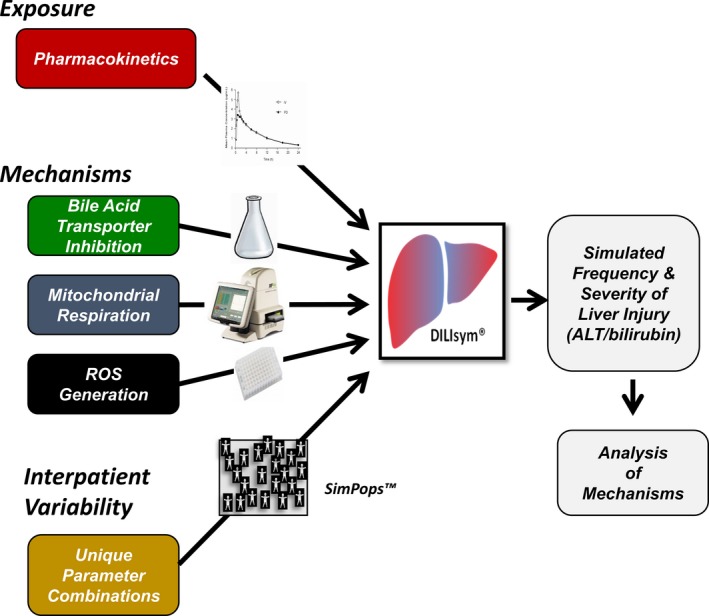
Application of the DILIsym model to predict hepatotoxicity. Extrahepatocyte and intrahepatocyte exposure to study drug is predicted by physiologically based pharmacokinetic modeling (see text). The three mechanisms listed are typically assessed from the dose‐dependent effects of drug and major metabolites on: (i) bile acid transporters using membrane vesicles, cell lines overexpressing transporters, or hepatocytes; (ii) mitochondrial respiration using the Seahorse instrument; and (iii) reactive oxygen species (ROS) generation measured with high content imaging. The collected exposure estimates and mechanistic data are put into the model, which will then predict the time‐dependent death of hepatocytes and, hence, the time‐dependent release of biomarkers into serum. Simulated patient populations have been created by changing parameters in the model to capture interpatient variation due to genetic or nongenetic factors. This permits estimates of the frequency as well as the extent of liver injury that can be anticipated in a real patient population receiving the drug. It is often possible to vary dosing and liver chemistry monitoring parameters to define protocols predicted to prevent serious DILI. ALT, alanine aminotransferase.
