Fig. 2.
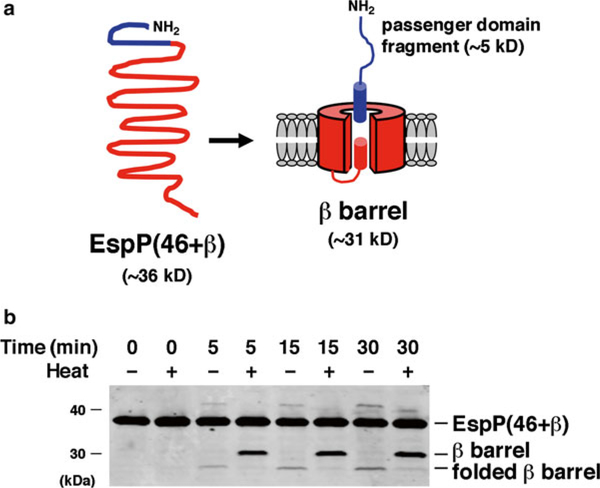
Assembly of a model OM protein catalyzed by the BAM complex and SurA. (a). EspP(46 + β) is a ~36 kD truncated form of the autotransporter EspP. Upon insertion into the OM EspP(46 + β) folds into a β-barrel structure. An α-helix traverses and protrudes through the extracellular side of the β-barrel pore. The protein is then rapidly cleaved into a 46-residue (~5 kD) “passenger domain” fragment and a ~31 kD β-barrel through an autocatayltic intrabarrel reaction. (b) EspP(46 + β) was incubated at 30 °C with proteoliposomes containing the BAM complex and SurA. Aliquots were removed at the indicated time points and either heated at 95 °C or maintained at room temperature. Proteins were resolved by SDS-PAGE and transferred to nitrocellulose. Western blotting was then conducted using an antiserum raised against a C-terminal EspP peptide [12]. Because proteolytic maturation requires membrane integration and folding of the protein, the accumulation of the free β-barrel demonstrates that a fraction of the protein was assembled during the incubation. The presence of a rapidly migrating form of the β-barrel in the absence of heat provides further evidence that it was properly folded. Part (b) was reproduced from Ref. 12 under a Creative Commons Attribution license