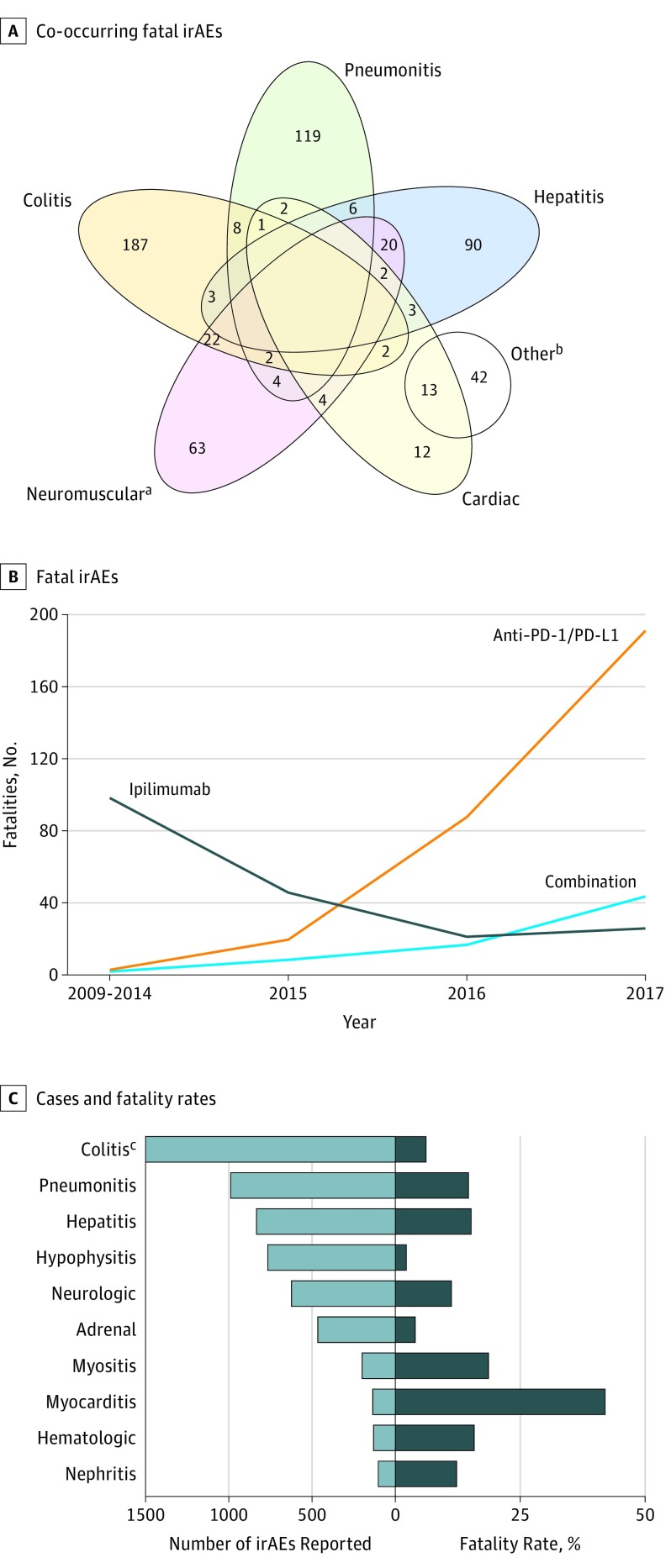
Figure 1. Clinical Characteristics of Fatal Immune-Related Adverse Events (irAEs).
A, Overlap of co-occurring fatal irAEs including colitis, pneumonitis, hepatitis, cardiac, and neuromuscular. B, Number of fatal irAEsreported by year and by immune checkpoint inhibitor regimen. C, Number of cases (light blue) and fatality rate (dark blue) for each class of toxic effect.
aOther group also contains: 14 patients with 2 other irAEs, 11 patients with a categorized irAE and 2 other irAEs, 3 patients with 3 other irAEs, 2 patients with 2 categorized irAEs and 2 other irAEs, 1 patient with 1 categorized irAE and 4 other irAEs.
bIncludes myasthenia gravis, myositis, and Guillain-Barre syndrome. All other concurrent irAEs (eg, noncardiac) are reflected in the Other group.
cIncludes 3905 total cases.