Abstract
Importance
Allogeneic blood or marrow transplantation (BMT) is a curative option for malignant and nonmalignant diseases of childhood. However, little is known about trends in cause-specific late mortality in this population during the past 3 decades.
Objectives
To examine cause-specific late mortality among individuals who have lived 2 years or more after allogeneic BMT performed in childhood and whether rates of late mortality have changed over time.
Design, Setting, and Participants
A retrospective cohort study was conducted of individuals who lived 2 years or more after undergoing allogeneic BMT performed in childhood between January 1, 1974, and December 31, 2010. The end of follow-up was December 31, 2016.
Exposure
Allogeneic BMT performed in childhood.
Main Outcomes and Measures
All-cause mortality, relapse-related mortality, and non–relapse-related mortality. Data on vital status and causes of death were collected using medical records, the National Death Index Plus Program, and Accurint databases.
Results
Among 1388 individuals (559 females and 829 males) who lived 2 years or more after allogeneic BMT performed in childhood, the median age at transplantation was 14.6 years (range, 0-21 years). In this cohort, there was a total of 295 deaths, yielding an overall survival rate of 79.3% at 20 years after BMT. The leading causes of death were infection and/or chronic graft-vs-host disease (121 of 244 [49.6%]), primary disease (60 of 244 [24.6%]), and subsequent malignant neoplasms (45 of 244 [18.4%]). Overall, the cohort had a 14.4-fold increased risk for death (95% CI, 12.8-16.1) compared with the general population (292 deaths observed; 20.3 deaths expected). Relative mortality remained elevated at 25 years or more after BMT (standardized mortality ratio, 2.9; 95% CI, 2.0-4.1). The absolute excess risk for death from any cause was 12.0 per 1000 person-years (95% CI, 10.5-13.5). The cumulative incidence of non–relapse-related mortality exceeded that of relapse-related mortality throughout follow-up. The 10-year cumulative incidence of late mortality decreased over time (before 1990, 18.9%; 1990-1999, 12.8%; 2000-2010, 10.9%; P = .002); this decrease remained statistically significant after adjusting for demographic and clinical factors (referent group: <1990; 1990-1999: hazard ratio, 0.64; 95% CI, 0.47-0.89; P = .007; 2000-2010: hazard ratio, 0.49; 95% CI, 0.31-0.76; P = .002; P < .001 for trend).
Conclusions and Relevance
Late mortality among children undergoing allogeneic BMT has decreased during the past 3 decades. However, these patients remain at an elevated risk of late mortality even 25 years or more after transplantation when compared with the general population, necessitating lifelong follow-up.
This cohort study examines cause-specific late mortality among individuals who have lived 2 years or more after allogeneic blood or marrow transplantation performed in childhood and whether rates of late mortality have changed over time.
Key Points
Question
What is the risk of late mortality after allogeneic blood or marrow transplantation performed in childhood, and has the rate of late mortality decreased over time?
Findings
In this cohort study of 1388 individuals who have lived 2 years or more after allogeneic blood or marrow transplantation performed in childhood, transplant recipients were at a continued elevated risk of premature death compared with the general population. Nonetheless, the rate of late mortality in this population has decreased during the past 3 decades.
Meaning
The risk of late mortality after allogeneic blood or marrow transplantation performed in childhood remains elevated for many years after transplantation, although the rate has decreased over time, and calls for lifelong proactive follow-up care.
Introduction
Allogeneic blood or marrow transplantation (BMT) is now a curative option for many malignant and nonmalignant diseases of childhood.1,2,3 Nonetheless, the high intensity of therapeutic exposures at a young age increases the risk of organ compromise and may lead to late mortality. Earlier studies of cohorts of adults who have undergone allogeneic BMT, as well as studies in mixed cohorts of both children and adults, have shown an increased risk of late mortality, primarily due to recurrent disease, subsequent malignant neoplasm, chronic graft-vs-host disease (cGvHD), infections, and cardiovascular and pulmonary disease.4,5,6,7,8,9,10 A few studies have examined late mortality after allogeneic BMT performed solely in childhood; these studies are limited by their relatively small sample sizes.11,12,13,14,15 Furthermore, it is not clear whether the mortality rates have changed during the past 3 decades in response to changes in transplant practice. We address these knowledge gaps by examining all-cause mortality, relapse-related mortality (RRM), and non-RRM in a large cohort of individuals who have lived 2 years or more after allogeneic BMT performed in childhood between 1974 and 2010.
Methods
The Blood or Marrow Transplant Survivor Study–2 is a collaborative effort between City of Hope, the University of Minnesota, and the University of Alabama at Birmingham examining the long-term outcome of individuals who have lived 2 years or more after BMT. To be included in this analysis, patients had to have received allogeneic BMT at City of Hope or the University of Minnesota between January 1, 1974, and December 31, 2010, at age 21 years or younger and lived for at least 2 years after transplantation. Patients who received a second BMT were excluded. The Human Subjects Committee at City of Hope and the University of Minnesota approved the Blood or Marrow Transplant Survivor Study–2 protocol. Written informed consent was obtained in accordance with the Declaration of Helsinki.16
Data on primary diagnosis, therapeutic agents used for preparative regimens, type of donor stem cells (related or unrelated donor), stem cell source, disease status at transplantation, and agents used for cGvHD prophylaxis, as well as demographic characteristics, were obtained on all eligible cases from the institutional transplant databases. National Death Index Plus17 and/or medical records provided data regarding the date and cause of death through December 31, 2015. Additional data from medical records and Accurint databases18 were used to extend the vital status information through December 31, 2016. All patients were assigned a primary and, if present, a secondary cause of death independently by 2 separate investigators (A.S.H. and J.W.). In the event of discrepant assignments, a third investigator (S.B.) provided adjudication. In the analyses of RRM and non-RRM, all patients with primary disease as a primary or secondary cause of death were assigned a relapse-related cause of death.
Statistical Analysis
Kaplan-Meier techniques were used to describe overall survival, conditional on living 2 years or more and 20 years or more after allogeneic BMT, as well as all-cause late mortality for patients receiving allogeneic BMT in 3 time periods: prior to 1990, 1990-1999, and 2000-2010. The standardized mortality ratio (SMR), a ratio of observed to expected number of deaths, was used to compare the mortality experienced by this cohort with the age-specific (5-year interval), sex-specific, and calendar-specific (5-year interval) mortality of the US general population. Person-years at risk were computed from the date 2 years after allogeneic BMT to either the date of death or the date of censoring (December 31, 2016), whichever occurred first. The expected number of deaths was calculated by multiplying the number of person-years in each defined stratum by the corresponding US mortality rates obtained from the Centers for Disease Control and Prevention.19 The Poisson regression method described by Vandenbroucke20 was used to calculate 95% CIs of the SMR. Absolute excess risk (AER) was defined as the difference between the observed and expected number of deaths per 1000 person-years of follow-up.
Cumulative incidence rates for RRM and non-RRM were calculated using competing risk methods. Cox proportional hazards regression analysis was used for identifying factors associated with all-cause mortality. The assumption of proportionality was tested, and all variables met the assumption (P > .05). A proportional subdistribution hazards (Fine-Gray) model for competing risks was used for identifying potential risk factors for RRM and non-RRM. The evaluated risk factors included age at allogeneic BMT, sex, race/ethnicity, primary disease, donor type, stem cell source, conditioning regimen, disease status at transplantation (standard or high risk of relapse at allogeneic BMT), and transplant time period. Mediation analysis was performed by adding each risk factor, one at time, to transplant time period, examining its association with the difference in late mortality across the transplant time periods; thus, using patients who underwent transplantation before 1990 as the referent group, we examined the hazard of late mortality for patients who underwent transplantation between 1990 and 1999 and those who underwent transplantation between 2000 and 2010 without adjustment for clinical or demographic factors. We then included each of these factors in separate models to examine if the hazard ratios (HRs) changed. Patients who underwent allogeneic BMT in the first or second complete remission after acute leukemia or lymphoma or in the first chronic phase of chronic myelogenous leukemia, as well as all patients with severe aplastic anemia, were considered at standard risk for relapse at transplantation; all other patients, excluding those already mentioned, were considered at high risk. Two-sided tests with P < .05 were considered statistically significant. All analyses were performed with SAS software, version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
The demographic and clinical characteristics of the cohort are shown in Table 1. In this cohort, 1388 children had undergone allogeneic BMT between 1974 and 2010 and lived at least 2 years. The median age at transplantation was 14.6 years (range, 0-21 years); 829 of the cohort were male patients (59.7%), 982 were non-Hispanic white patients (70.7%), and 658 of patients had undergone transplantation (47.4%) between 2000 and 2010. The most common primary diagnoses were acute lymphoblastic leukemia (348 patients [25.1%]), acute myeloid leukemia or myelodysplastic syndrome (326 patients [23.5%]), inborn errors of metabolism (192 patients [13.8%]), and severe aplastic anemia ([SAA] 147 patients [10.6%]). A total of 642 patients (46.3%) had a disease that was at high risk of relapse at transplantation. The majority of patients (803 [57.9%]) had received stem cells from a related donor, and bone marrow (1019 [73.4%]) was the major source of stem cells. Total body irradiation was used as part of the conditioning regimen for 892 patients (64.3%), cyclophosphamide was used for 1118 patients (80.5%), and busulfan was used for 355 patients (25.6%).
Table 1. Demographic and Clinical Characteristics of Individuals Who Lived 2 Years or More After Allogeneic BMT Performed in Childhood.
| Variable | Patients, No. (%) (N = 1388) |
|---|---|
| Treating institution | |
| University of Minnesota | 950 (68.4) |
| City of Hope | 438 (31.6) |
| Sex | |
| Male | 829 (59.7) |
| Female | 559 (40.3) |
| Race/ethnicity | |
| Non-Hispanic white | 982 (70.7) |
| Hispanic | 213 (15.3) |
| Non-Hispanic black | 71 (5.1) |
| Other | 96 (6.9) |
| Unknown | 26 (1.9) |
| Age at BMT, y | |
| ≥4 | 379 (27.3) |
| 5-9 | 362 (26.1) |
| 10-14 | 266 (19.2) |
| 15-21 | 381 (27.4) |
| Time period of BMT | |
| <1990 | 323 (23.3) |
| 1990-1999 | 407 (29.3) |
| 2000-2010 | 658 (47.4) |
| Type of donor | |
| Related | 803 (57.9) |
| Unrelated | 585 (42.1) |
| Source of stem cells | |
| Bone marrow | 1019 (73.4) |
| Cord blood | 253 (18.2) |
| PBSCs | 116 (8.4) |
| Primary disease | |
| ALL | 348 (25.1) |
| AML or MDS | 326 (23.5) |
| Inborn errors of metabolism | 192 (13.8) |
| Severe aplastic anemia | 147 (10.6) |
| Fanconi anemiaa | 115 (8.3) |
| Chronic myelogenous leukemia | 90 (6.5) |
| Immune disorders | 55 (4.0) |
| Sickle cell disease or thalassemia | 26 (1.9) |
| Other malignant diseaseb | 64 (4.6) |
| Other nonmalignant diseasec | 25 (1.8) |
| Conditioning regimen | |
| Cyclophosphamide | 1118 (80.5) |
| Total body irradiation | 892 (64.3) |
| Antithymocyte globulin | 563 (40.6) |
| Busulfan | 355 (25.6) |
| Fludarabine | 251 (18.1) |
| Etoposide | 216 (15.6) |
| Melphalan | 68 (4.9) |
| Cytarabine | 58 (4.2) |
| Other chemotherapy | 126 (9.1) |
| Other radiotherapy | 84 (6.1) |
| Total body irradiation plus cyclophosphamide | 691 (49.8) |
| Busulfan plus cyclophosphamide | 326 (23.5) |
| Disease status at BMT | |
| Standard risk of relapsed | 742 (53.5) |
| High risk of relapse | 642 (46.3) |
| Chronic GvHD prophylaxis | |
| Yes | 1361 (98.1) |
| Cyclosporine | 953 (68.7) |
| Methotrexate | 777 (56.0) |
| Systemic corticosteroids | 582 (41.9) |
| Mycophenolic acid | 206 (14.8) |
| T-cell depletion | 190 (13.7) |
| Tacrolimus or sirolimus | 99 (7.1) |
| No. of deaths | 295 (21.3) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMT, blood or marrow transplantation; GvHD, graft-vs-host disease; MDS, myelodysplastic syndrome; PBSC, peripheral blood stem cell.
Includes Fanconi anemia, Diamond Blackfan anemia, and Shwachman-Diamond syndrome.
Includes 27 patients with non-Hodgkin lymphoma, 7 with Hodgkin lymphoma, 8 with neuroblastoma, 2 with unspecified leukemia, and 20 with histiocytosis.
Includes 1 patient with megakaryocytosis, 3 with dyskeratotis congenita, 8 with epidermolysis bullosa, 1 with Kostmann agranulocytosis, 11 with osteopetrosis, and 1 with paroxysmal nocturnal hemoglobinuria.
Standard risk: first or second complete remission after acute leukemia or lymphoma or first chronic phase of chronic myelogenous leukemia or severe aplastic anemia; all others, high risk.
Overall Survival
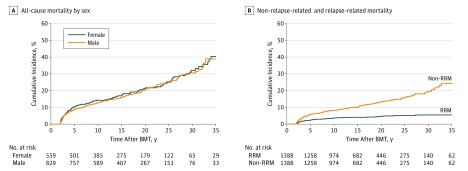
After a median follow-up of 14.9 years (range, 2.0-41.2 years), 295 patients had died at a median age of 20.8 years, yielding an overall survival rate of 79.3% at 20 years after allogeneic BMT. Conditional on living the first 2 years after BMT, the 20-year overall survival rate by primary diagnosis was 92.4% for immune disorders, 91.0% for SAA, 82.3% for sickle cell anemia or thalassemia, 81.1% for acute myeloid leukemia or myelodysplastic syndrome, 77.2% for chronic myelogenous leukemia, 76.1% for inborn errors of metabolism, 72.7% for acute lymphoblastic leukemia, and 68.5% for Fanconi anemia. Sex-stratified cumulative all-cause mortality for the entire cohort is shown in Figure 1A, revealing no difference in survival by sex. Conditional on living 20 years after BMT, the subsequent 5-year overall survival was 94.2% for the entire cohort (eTable 1 in the Supplement).
Figure 1. Cumulative All-Cause Mortality of 1388 Individuals Who Lived 2 Years or More After Allogeneic Blood or Marrow Transplantation (BMT) Performed in Childhood.
A, Cumulative all-cause mortality by sex at 10 years (female patients, 14.3%; male patients, 12.8%; P = .78). B, Cumulative non–relapse-related mortality (non-RRM) at 20 years (13.2%) and relapse-related mortality (RRM) at 20 years (4.5%).
Relative Mortality
Table 2 summarizes the SMRs in this population using age-specific, sex-specific, and calendar-specific rates from the general US population. Overall, the cohort was at a 14.4-fold increased risk for premature death (95% CI, 12.8-16.1) compared with the general population (292 deaths observed; 20.3 deaths expected). Relative mortality was elevated across all ages at transplantation but was highest for patients who underwent transplantation at age 5 to 9 years (SMR, 22.8; 95% CI, 17.9-28.4). The SMRs were higher for female patients (23.6; 95% CI, 19.6-28.0) compared with male patients (11.2; 95% CI, 9.6-13.0; P < .001). The SMRs were highest for patients who underwent transplantation in the most recent time period (20.8; 95% CI, 16.4-25.9), likely owing to the low mortality rates among the corresponding children in the general population. The SMRs were higher among those who underwent transplantation for disease at high risk of relapse (23.3; 95% CI, 21.2-25.6) and those who had received an unrelated donor BMT (19.9; 95% CI, 16.0-24.3). High relative mortality was observed across all primary diagnoses, with SMRs ranging from 4.6 (SAA) to 28.2 (inborn errors of metabolism). Relative mortality was highest in the 2- to 5-year period after BMT (SMR, 522.0; 95% CI, 439.9-613.6) but decreased rapidly thereafter. However, it remained significantly elevated even at 25 years or more after transplantation (SMR, 2.9; 95% CI, 2.0-4.1).
Table 2. SMR and AER Among 1388 Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood.
| Variable | Entire Cohort, No. of Patients | All-Cause Mortality (95% CI) | |
|---|---|---|---|
| SMR | AER | ||
| All patients | 1388 | 14.4 (12.8 to 16.1) | 12.0 (10.5 to 13.5) |
| Sex | |||
| Male | 829 | 11.2 (9.6 to 13.0) | 11.5 (9.6 to 13.4) |
| Female | 559 | 23.6 (19.6 to 28.0) | 12.7 (10.4 to 15.1) |
| Age at BMT, y | |||
| ≤4 | 379 | 20.3 (15.8 to 25.7) | 9.6 (7.1 to 12.0) |
| 5-9 | 362 | 22.8 (17.9 to 28.4) | 12.9 (9.8 to 16.0) |
| 10-14 | 266 | 12.8 (9.8 to 16.3) | 11.9 (8.6 to 15.2) |
| 15-21 | 381 | 10.3 (8.4 to 12.5) | 13.8 (10.8 to 16.9) |
| Time period of BMT | |||
| <1990 | 323 | 12.6 (10.6 to 14.9) | 15.0 (12.3 to 17.8) |
| 1990-1999 | 407 | 13.6 (10.9 to 16.6) | 10.3 (7.9 to 12.6) |
| 2000-2010 | 658 | 20.8 (16.4 to 25.9) | 10.4 (7.9 to 12.9) |
| Type of donor | |||
| Related | 803 | 12.8 (11.1 to 14.7) | 12.6 (10.7 to 14.4) |
| Unrelated | 585 | 19.9 (16.0 to 24.3) | 11.0 (8.6 to 13.3) |
| Source of stem cells | |||
| Bone marrow | 1019 | 13.4 (11.8 to 15.2) | 12.1 (10.4 to 13.7) |
| Cord blood | 253 | 21.2 (14.3 to 30.0) | 9.3 (5.7 to 12.8) |
| PBSC | 116 | 22.5 (14.5 to 32.9) | 17.8 (10.2 to 25.4) |
| Primary diagnosis | |||
| ALL | 348 | 18.5 (15.1 to 22.4) | 17.3 (13.7 to 20.9) |
| AML or MDS | 326 | 13.3 (10.5 to 16.7) | 11.6 (8.6 to 14.5) |
| Inborn errors of metabolism | 192 | 28.2 (20.6 to 37.5) | 14.6 (10.1 to 19.2) |
| Severe aplastic anemia | 147 | 4.6 (2.8 to 7.0) | 4.3 (1.8 to 6.9) |
| Fanconi anemiaa | 115 | 21.0 (11.6 to 34.6) | 9.3 (4.0 to 14.6) |
| Chronic myelogenous leukemia | 90 | 11.8 (7.3 to 17.9) | 11.5 (5.8 to 17.1) |
| Other malignant diseaseb | 64 | 14.0 (7.9 to 22.7) | 13.8 (6.0 to 21.6) |
| Immune disorders | 55 | 9.3 (4.0 to 18.0) | 5.6 (1.0 to 10.2) |
| Sickle cell disease or thalassemia | 26 | 24.1 (6.0 to 62.5) | 9.1 (–1.6 to 19.7) |
| Other nonmalignant diseasec | 25 | 37.9 (15.1 to 76.8) | 19.9 (3.6 to 36.3) |
| Disease status at BMT | |||
| Standard risk of relapsed | 742 | 10.3 (9.4 to 11.3) | 9.9 (8.1 to 11.7) |
| High risk of relapse | 642 | 23.3 (21.2 to 25.6) | 15.1 (12.6 to 17.6) |
| Overall survival after BMT, y | |||
| 2-5 | 142 | 522.0 (439.9 to 613.6) | 310.8 (259.0 to 362.6) |
| 6-9 | 337 | 35.9 (26.3 to 47.6) | 15.0 (10.5 to 19.6) |
| 10-14 | 281 | 14.5 (10.0 to 20.0) | 7.9 (5.0 to 10.9) |
| 15-19 | 218 | 9.1 (6.1 to 13.0) | 6.0 (3.5 to 8.5) |
| 20-24 | 164 | 5.8 (3.6 to 8.8) | 4.3 (2.0 to 6.6) |
| ≥25 | 246 | 2.9 (2.0 to 4.1) | 2.6 (1.2 to 3.9) |
Abbreviations: AER, absolute excess risk; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMT, blood or marrow transplantation; MDS, myelodysplastic syndrome; PBSC, peripheral blood stem cells; SMR, standardized mortality ratio.
Includes Fanconi anemia, Diamond Blackfan anemia, and Shwachman-Diamond syndrome.
Includes 27 patients with non-Hodgkin lymphoma, 7 with Hodgkin lymphoma, 8 with neuroblastoma, 2 with unspecified leukemia, and 20 with histiocytosis.
Includes 1 patient with megakaryocytosis, 3 with dyskeratotis congenita, 8 with epidermolysis bullosa, 1 with Kostmann agranulocytosis, 11 with osteopetrosis, and 1 with paroxysmal nocturnal hemoglobinuria.
First or second complete remission after acute leukemia or lymphoma or first chronic phase of chronic myelogenous leukemia or severe aplastic anemia; all others, high risk.
The AER for death from any cause for the whole cohort was 12.0 per 1000 person-years (95% CI, 10.5-13.5). The AERs did not vary significantly by sex, age at BMT, type of donor, or transplant time period (Table 2). The AERs were higher for those with disease that was at high risk of relapse (15.1; 95% CI, 12.6-17.6) than those with disease at standard risk of relapse (9.9; 95% CI, 8.1-11.7). The AER was highest in the period between 2 and 5 years after BMT (310.8; 95% CI, 259.0-362.6) and decreased progressively thereafter, but it was significantly elevated among those who had lived 25 years or more after BMT (2.6; 95% CI, 1.2-3.9).
Specific Causes of Death
A cause of death was available for 244 of the 295 deceased patients (82.7%), in part because we had National Death Index Plus data through 2015 and vital status data through 2016. The causes of late death included infection and/or cGvHD (121 [49.6%]), primary disease (60 [24.6%]), subsequent malignant neoplasm (45 [18.4%]), cardiac disease (24 [9.8%]), pulmonary disease (19 [7.8%]), external causes (7 [2.9%]), and other miscellaneous causes (44 [18.0%]). The cumulative incidence of cause-specific mortality is shown in eFigure 1 in the Supplement. The 10-year cumulative incidence of the most prevalent causes of death was as follows: 4.4% (95% CI, 3.4%-5.6%) for infection; 2.5% (95% CI, 1.7%-3.4%) for cGvHD; and 1.6% (95% CI, 1.0%-2.4%) for subsequent malignant neoplasm (eTable 2 in the Supplement). A total of 49 of the 60 deaths due to primary disease (81.7%) occurred within the first 2 to 9 years after transplantation. The cumulative incidence of non-RRM was 13.2% at 20 years after BMT, while that of RRM was only 4.5% (Figure 1B).
Factors Associated With Late Mortality
Table 3 summarizes the results from the multivariable regression analysis of all-cause late mortality, RRM, and non-RRM. Sex, race/ethnicity, or donor type (related vs unrelated) were not associated with all-cause late mortality. The hazard of all-cause late mortality increased with age at BMT (HR, 1.03; 95% CI; 1.01-1.05) and was higher among those who underwent transplantation for disease at high risk of relapse (HR, 1.95; 95% CI, 1.45-2.63) compared with those who underwent transplantation for disease with standard risk of relapse. On the other hand, compared with those who underwent transplantation for acute lymphoblastic leukemia, the hazard of all-cause late mortality was lower among those who underwent transplantation for acute myeloid leukemia or myelodysplastic syndrome (HR, 0.72; 95% CI, 0.53-0.99), chronic myelogenous leukemia (HR, 0.53; 95% CI, 0.32-0.88), Fanconi anemia (HR, 0.49; 95% CI, 0.26-0.94), immune disorders (HR, 0.32; 95% CI, 0.14-0.74), and SAA (HR, 0.33; 95% CI; 0.19-0.57). Furthermore, the hazard of all-cause late mortality was significantly lower among those who received busulfan and cyclophosphamide as a conditioning regimen (HR, 0.62; 95% CI, 0.41-0.95) compared with those who received total body irradiation and cyclophosphamide.
Table 3. Adjusted HRs of Late All-Cause, Relapse-Related, and Non–Relapse-Related Mortality Among 1388 Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood.
| Variable | All-Cause Mortality | Relapse-Related Mortality | Non–Relapse-Related Mortality | |||
|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| Sex | ||||||
| Male | 0.93 (0.74-1.18) | .60 | 1.26 (0.74-2.25) | .40 | 0.94 (0.70-1.27) | .70 |
| Female | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Non-Hispanic black | 1.53 (0.91-2.57) | .10 | 0.75 (0.11-2.66) | .70 | 1.62 (0.81-3.01) | .10 |
| Hispanic | 0.86 (0.59-1.25) | .40 | 0.64 (0.23-1.48) | .30 | 1.00 (0.63-1.56) | .99 |
| Other | 1.24 (0.78-1.95) | .40 | 1.03 (0.31-2.62) | >.99 | 1.30 (0.71-2.21) | .40 |
| Unknown | 0.30 (0.04-2.17) | .20 | NA | NA | 0.51 (0.03-2.37) | .50 |
| Age at BMT, ya | 1.03 (1.01-1.05) | .004 | 1.03 (0.98-1.07) | .20 | 1.03 (1.00-1.06) | .03 |
| Year of BMT | ||||||
| <1990 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| 1990-1999 | 0.64 (0.47-0.89) | .008 | 0.74 (0.38-1.43) | .40 | 0.67 (0.44-1.01) | .06 |
| 2000-2010 | 0.49 (0.31-0.76) | .002 | 0.67 (0.28-1.51) | .30 | 0.46 (0.25-0.80) | .01 |
| Type of donor | ||||||
| Related | 1 [Reference] | |||||
| Unrelated | 1.06 (0.76-1.70) | .74 | 0.71 (0.34-1.43) | .40 | 1.30 (0.86-1.95) | .20 |
| Source of stem cells | ||||||
| Bone marrow | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Cord blood | 0.83 (0.52-1.34) | .50 | 0.67 (0.22-1.82) | .40 | 0.96 (0.53-1.68) | .90 |
| PBSC | 1.77 (1.01-3.11) | .05 | 0.96 (0.24-3.14) | .90 | 2.39 (1.21-4.65) | .01 |
| Primary diagnosis | ||||||
| ALL | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| AML or MDS | 0.72 (0.53-0.99) | .04 | 0.39 (0.18-0.80) | .01 | 0.79 (0.53-1.16) | .20 |
| Inborn errors of metabolism | 0.98 (0.61-1.57) | .90 | 1.27 (0.49-3.28) | .60 | 0.84 (0.45-1.52) | .50 |
| Severe aplastic anemia | 0.33 (0.19-0.57) | <.001 | 0.09 (0.01-0.48) | .03 | 0.36 (0.17-0.71) | .004 |
| Fanconi anemiab | 0.49 (0.26-0.94) | .03 | NA | NA | 0.70 (0.32-1.43) | .40 |
| Chronic myelogenous leukemia | 0.53 (0.32-0.88) | .02 | 0.24 (0.04-0.85) | .06 | 0.60 (0.31-1.08) | .10 |
| Other malignant diseasec | 0.70 (0.39-1.25) | .20 | 0.87 (0.25-2.35) | .80 | 0.82 (0.39-1.58) | .60 |
| Immune disorders | 0.32 (0.14-0.74) | .006 | 0.24 (0.01-1.38) | .20 | 0.14 (0.02-0.48) | .009 |
| Sickle cell disease or thalassemia | 0.46 (0.13-1.56) | .20 | 0.74 (0.04-4.97) | .80 | 0.47 (0.07-1.72) | .30 |
| Other nonmalignant diseased | 1.03 (0.43-2.44) | >.99 | 0.71 (0.04-3.90) | .70 | 0.79 (0.19-2.26) | .70 |
| Disease status at BMT | ||||||
| Standard risk of relapsee | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| High risk of relapse | 1.95 (1.45-2.63) | <.001 | 1.57 (0.80-2.99) | .20 | 2.05 (1.41-2.94) | <.001 |
| Conditioning regimen | ||||||
| Total body irradiation plus cyclophosphamide | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Busulfan plus cyclophosphamide | 0.62 (0.41-0.95) | .03 | 0.63 (0.27-1.47) | .30 | 0.71 (0.41-1.19) | .20 |
| Others | 0.88 (0.64-1.21) | .40 | 0.73 (0.36-1.44) | .30 | 0.93 (0.62-1.38) | .70 |
Abbreviation: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMT, blood or marrow transplantation; MDS, myelodysplastic syndrome; HR, hazard ratio; NA, not applicable; PBSC, peripheral blood stem cells.
Age included in the model as a continuous variable.
Includes Fanconi anemia, Diamond Blackfan anemia, and Shwachman-Diamond syndrome.
Includes 27 with non-Hodgkin lymphoma, 7 with Hodgkin lymphoma, 8 with neuroblastoma, 2 with unspecified leukemia, and 20 with histiocytosis.
Includes 1 with megakaryocytosis, 3 with dyskeratotis congenita, 8 with epidermolysis bullosa, 1 with Kostmann agranulocytosis, 11 with osteopetrosis, and 1 with paroxysmal nocturnal hemoglobinuria.
First or second complete remission after acute leukemia or lymphoma or first chronic phase of chronic myelogenous leukemia or severe aplastic anemia, all others high risk.
Compared with patients who underwent transplantation for acute lymphoblastic leukemia, the hazard of RRM was lower among patients who underwent transplantation for acute myeloid leukemia or myelodysplastic syndrome (HR, 0.39; 95% CI, 0.18-0.80); on the other hand, the hazard of non-RRM was lower among those who underwent transplantation for SAA (HR, 0.36; 95% CI, 0.17-0.71) and immune disorders (HR, 0.14; 95% CI, 0.02-0.48) (Table 3). The hazard of non-RRM increased with age at BMT (HR, 1.03; 95% CI, 1.00-1.06) and was higher among those who received peripheral blood stem cells compared with those who received bone marrow (HR, 2.39; 95% CI, 1.21-4.65) and was high among those who were at a higher risk of relapse at BMT (HR, 2.05; 95% CI, 1.41-2.94).
Late Mortality by Treatment Time Period
The demographic and clinical characteristics of the present cohort by treatment time period are detailed in eTable 3 in the Supplement, and noteworthy differences are illustrated in eFigure 2 in the Supplement. There was a decrease in the proportion of non-Hispanic white individuals undergoing BMT, with a concomitant increase in the proportion of African American and Hispanic individuals. There was an increase in the number of unrelated donor BMTs and an increase in the number of peripheral blood stem cell transplants and cord blood stem cell transplants. Use of total body irradiation decreased. The number of patients who underwent transplantation with high-risk disease increased.
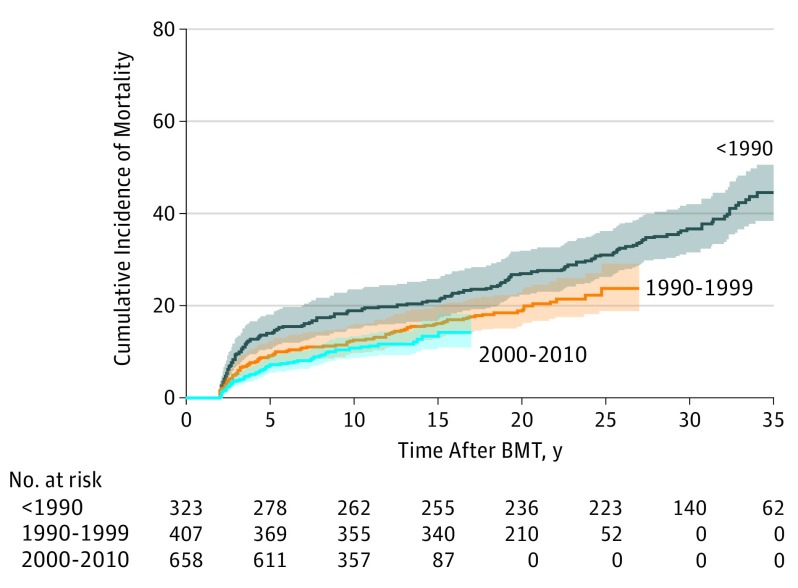
The all-cause, 10-year cumulative mortality rate decreased during the 3 time periods (18.9% prior to 1990, 12.9% in 1990-1999, and 11.0% in 2000-2010; P = .002) (Figure 2). Adjusting for demographic and clinical variables (including all the variables that had changed significantly during the 3 time periods), the HR of all-cause death decreased with time (referent group, prior to 1990; 1990-1999: HR, 0.64; 95% CI; 0.47-0.89; P = .007; 2000-2010: HR, 0.49; 95% CI, 0.31-0.76; P = .002; P < .001 for trend) (Table 3). As shown in Table 3, the adjusted hazard of non-RRM decreased significantly during the treatment time periods; however, the decrease in RRM did not approach statistical significance. Mediation analysis, including clinical transplant practice variables as well as demographic factors, did not explain the decrease in late mortality over time (eTable 4 in the Supplement).
Figure 2. Cumulative All-Cause Mortality of 1388 Individuals Who Lived 2 Years or More After Allogeneic Blood or Marrow Transplantation (BMT) Performed in Childhood, by Treatment Time Period.
Cumulative incidence of mortality at 10 years (18.9% prior to 1990, 12.9% in 1990-1999, and 11.0% in 2000-2010; P = .002). The shaded areas indicate 95% CIs.
Discussion
This study on late mortality among individuals who lived 2 years or more after allogeneic BMT performed in childhood shows that these patients remain at an elevated risk of premature death compared with the general population even 25 years after transplantation. Although the risk of RRM plateaus with time, non-RRM continues to increase and remains the major cause of excess late death in this population. Most important, there has been a significant decrease in all-cause mortality experienced by children undergoing allogeneic BMT during the past 3 decades.
The present study demonstrates that patients who live 2 or more years after allogeneic BMT performed at age 21 years or younger have an overall survival rate that approaches 80% at 20 years after transplantation. This study also demonstrates that this cohort is at a 14-fold increased risk of premature death compared with the general population. The mortality rate continues to be significantly higher among those who live 25 or more years after transplantation. An Australian cohort of individuals who lived 2 years or more after pediatric allogeneic BMT had an SMR of 35.9 compared with the general population.13 However, unlike our study, the Australian cohort included only malignant disorders.
Similar to the previously published Australian study,13 we found that the SMRs were highest among those who underwent transplantation at a young age, likely owing to the negligible death rates at this age in the general population. However, we found that the AER was lowest among those who underwent transplantation at 4 years or younger and was relatively similar across the other age groups. In the present study, high relative mortality was observed across all primary diagnoses, with the highest rates among individuals with a primary diagnosis of inborn errors of metabolism, Fanconi anemia, and acute lymphoblastic leukemia. The SMR among individuals who underwent transplantation for SAA was considerably lower. Among individuals who lived 2 years or more after allogeneic BMT for inborn errors of metabolism, a previous report found a 7-year overall survival rate of 90%, which is comparable to our study.21 The lower relative mortality rates among individuals who lived 2 years or more after allogeneic BMT for SAA are in concordance with previously published results.4,9 The cumulative all-cause mortality and the AER did not differ significantly between the sexes, although a higher SMR was found among female patients rather than male patients. However, the latter finding can likely be explained by higher mortality rates among younger male patients in the general population.
We show for the first time, to our knowledge, that while the risk of RRM plateaus with time, non-RRM continues to increase and remains the major cause of late death in this population. In our study, only 25% of the late deaths were caused by the primary disease, while 65% died of relapse in the study by Schechter et al.12 Again, the latter study was smaller and restricted to malignant diseases. The proportion of late deaths attributed to primary disease was also higher in the Australian report that included only malignant diseases (73% [32 deaths]).13 However, the proportions of late deaths due to primary disease in larger studies, although based on mixed cohorts of adults and children treated with allogeneic BMT for malignant diseases, are more comparable to ours.4,5,7 Similar to the pattern observed in the present study, Wingard et al4 demonstrated a distinct pattern of a decreasing share of primary diagnosis causing death with time from transplant.
In the present study, we found that there has been a significant decrease in all-cause late mortality among children undergoing allogeneic BMT during the past 3 decades. Although there were significant changes in transplant practice during these 3 time periods, a mediation analysis including each of these transplant practices in the model failed to explain the decrease in mortality, suggesting that perhaps unmeasured variables (such as supportive care strategies, management of cGvHD, or improved patient selection for transplantation) could explain the decrease in late mortality.
In accordance with the existing literature on cause-specific late mortality after allogeneic BMT,4,5,7,10 primary disease, infection, subsequent malignant neoplasm, and cGvHD were the most common causes of death in this study. Chronic graft-vs-host disease and infection led to death primarily in the earlier part of follow-up, with 79% (cGvHD) and 78% (infection) occurring within the first 2 to 9 years after transplant. These findings underscore the need for close follow-up with regard to cGvHD and infections, in addition to relapse, during the first decade after transplantation. Subsequent malignant neoplasms accounted for 18% of the deaths observed in this study. Subsequent malignant neoplasms have also been reported as a significant cause of late mortality among adults undergoing allogeneic BMT.4,5,7 Taken together, our results clearly show that long-term follow-up of individuals who lived 2 years or more after allogeneic BMT in childhood needs to include screening, preventive interventions, and counseling throughout life.
Limitations
Similar to the present study, most studies on cause-specific late mortality are based on causes of death registered on death certificates. Given the limitations of death certificates, some degree of misclassification is unavoidable.5,22,23 Striving to partly overcome this bias, 3 of us (A.S.H., J.W., and S.B.) reviewed the causes of death recorded on the death certificates. Furthermore, information about the causes of death was lacking for 51 of the 295 deceased patients (17.3%). This study was also limited by the lack of data on pretransplant treatment.
Conclusions
These limitations notwithstanding, this study demonstrates that there has been a significant decrease in all-cause late mortality among children undergoing allogeneic BMT during the past 3 decades. Nevertheless, these individuals who lived 2 years or more after BMT remain at an elevated risk of late death even 25 years after BMT. Although the risk of RRM plateaus with time, non-RRM continues to increase and is the major cause of late death in this population. These findings emphasize the need for lifelong follow-up care after allogeneic BMT performed in childhood, focusing on the need for surveillance and early management of infections, cGvHD, and disease recurrence during the first decade after transplantation, as well as screening for early detection of subsequent malignant neoplasms and other complications throughout life.
eFigure 1. Cumulative Incidence of Cause-Specific Mortality of Individuals Who Lived 2 Years or More After Allogenic BMT Performed in Childhood
eFigure 2. Demographic and Treatment Characteristics of Individuals Who Lived 2 Years or More After Allogenic BMT Performed in Childhood, by Treatment Era
eTable 1. Conditional Survival in Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood, by Primary Diagnosis
eTable 2. Cumulative Incidence of Mortality Among Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood, by Cause of Death
eTable 3. Demographic and Treatment Characteristics of Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood, by Treatment Era
eTable 4. Mediation Analysis of Hazard of All-Cause Mortality by Treatment Era Among Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood
References
- 1.D’Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2017;23(9):-. doi: 10.1016/j.bbmt.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandelwal P, Millard HR, Thiel E, et al. . Hematopoietic stem cell transplantation activity in pediatric cancer between 2008 and 2014 in the United States: a Center for International Blood and Marrow Transplant research report. Biol Blood Marrow Transplant. 2017;23(8):1342-1349. doi: 10.1016/j.bbmt.2017.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miano M, Labopin M, Hartmann O, et al. ; Paediatric Diseases Working Party of the European Group for Blood and Marrow Transplantation . Haematopoietic stem cell transplantation trends in children over the last three decades: a survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2007;39(2):89-99. doi: 10.1038/sj.bmt.1705550 [DOI] [PubMed] [Google Scholar]
- 4.Wingard JR, Majhail NS, Brazauskas R, et al. . Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230-2239. doi: 10.1200/JCO.2010.33.7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin PJ, Counts GW Jr, Appelbaum FR, et al. . Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011-1016. doi: 10.1200/JCO.2009.25.6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socié G, Stone JV, Wingard JR, et al. ; Late Effects Working Committee of the International Bone Marrow Transplant Registry . Long-term survival and late deaths after allogeneic bone marrow transplantation. N Engl J Med. 1999;341(1):14-21. doi: 10.1056/NEJM199907013410103 [DOI] [PubMed] [Google Scholar]
- 7.Bhatia S, Francisco L, Carter A, et al. . Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784-3792. doi: 10.1182/blood-2007-03-082933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pond GR, Lipton JH, Messner HA. Long-term survival after blood and marrow transplantation: comparison with an age- and gender-matched normative population. Biol Blood Marrow Transplant. 2006;12(4):422-429. doi: 10.1016/j.bbmt.2005.11.518 [DOI] [PubMed] [Google Scholar]
- 9.Vajdic CM, Mayson E, Dodds AJ, et al. ; CAST study investigators . Second cancer risk and late mortality in adult Australians receiving allogeneic hematopoietic stem cell transplantation: a population-based cohort study. Biol Blood Marrow Transplant. 2016;22(5):949-956. doi: 10.1016/j.bbmt.2016.01.027 [DOI] [PubMed] [Google Scholar]
- 10.Atsuta Y, Hirakawa A, Nakasone H, et al. ; Late Effect and Quality of Life Working Group of the Japan Society for Hematopoietic Cell Transplantation . Late mortality and causes of death among long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(9):1702-1709. doi: 10.1016/j.bbmt.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 11.Wilhelmsson M, Vatanen A, Borgström B, et al. . Adverse health events and late mortality after pediatric allogeneic hematopoietic SCT—two decades of longitudinal follow-up. Bone Marrow Transplant. 2015;50(6):850-857. doi: 10.1038/bmt.2015.43 [DOI] [PubMed] [Google Scholar]
- 12.Schechter T, Pole JD, Darmawikarta D, et al. . Late mortality after hematopoietic SCT for a childhood malignancy. Bone Marrow Transplant. 2013;48(10):1291-1295. doi: 10.1038/bmt.2013.64 [DOI] [PubMed] [Google Scholar]
- 13.Nelson AS, Ashton LJ, Vajdic CM, et al. ; CAST study investigators . Second cancers and late mortality in Australian children treated by allogeneic HSCT for haematological malignancy. Leukemia. 2015;29(2):441-447. doi: 10.1038/leu.2014.203 [DOI] [PubMed] [Google Scholar]
- 14.Nelson AS, Vajdic CM, Ashton LJ, et al. ; CAST investigators . Incident cancers and late mortality in Australian children treated by allogeneic stem cell transplantation for non-malignant diseases. Pediatr Blood Cancer. 2017;64(1):197-202. doi: 10.1002/pbc.26219 [DOI] [PubMed] [Google Scholar]
- 15.Ferry C, Gemayel G, Rocha V, et al. . Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. 2007;40(3):219-224. doi: 10.1038/sj.bmt.1705710 [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention National Death Index. https://www.cdc.gov/nchs/ndi/index.htm. Accessed Jan 19, 2018.
- 18.LexisNexis Accurint. http://www.accurint.com. Accessed Jan 19, 2018.
- 19.Centers for Disease Control and Prevention Compressed mortality file: underlying cause-of-death. https://wonder.cdc.gov/mortSQL.html. Accessed January 19, 2018.
- 20.Vandenbroucke JP. A shortcut method for calculating the 95 per cent confidence interval of the standardized mortality ratio. Am J Epidemiol. 1982;115(2):303-304. doi: 10.1093/oxfordjournals.aje.a113306 [DOI] [Google Scholar]
- 21.Eapen M, Ahn KW, Orchard PJ, et al. . Long-term survival and late deaths after hematopoietic cell transplantation for primary immunodeficiency diseases and inborn errors of metabolism. Biol Blood Marrow Transplant. 2012;18(9):1438-1445. doi: 10.1016/j.bbmt.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Möller TR, Garwicz S, Barlow L, et al. ; Association of the Nordic Cancer Registries; Nordic Society for Pediatric Hematology and Oncology . Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol. 2001;19(13):3173-3181. doi: 10.1200/JCO.2001.19.13.3173 [DOI] [PubMed] [Google Scholar]
- 23.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med. 2001;161(2):277-284. doi: 10.1001/archinte.161.2.277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Cumulative Incidence of Cause-Specific Mortality of Individuals Who Lived 2 Years or More After Allogenic BMT Performed in Childhood
eFigure 2. Demographic and Treatment Characteristics of Individuals Who Lived 2 Years or More After Allogenic BMT Performed in Childhood, by Treatment Era
eTable 1. Conditional Survival in Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood, by Primary Diagnosis
eTable 2. Cumulative Incidence of Mortality Among Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood, by Cause of Death
eTable 3. Demographic and Treatment Characteristics of Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood, by Treatment Era
eTable 4. Mediation Analysis of Hazard of All-Cause Mortality by Treatment Era Among Individuals Who Lived 2 Years or More After Allogeneic BMT in Childhood