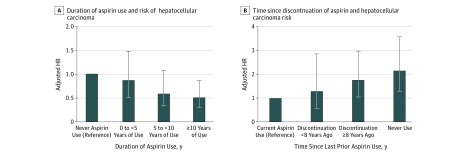
Figure. Hepatocellular Carcinoma Risk in Relation to Duration of Aspirin Use and Time Since Aspirin Discontinuation.
A, Duration of aspirin use and hepatocellular carcinoma risk. Categories of aspirin use duration were compared with individuals reporting never-use of aspirin (reference). The P value for trend was calculated using continuous duration of use (months) among aspirin users, compared with the lowest reported duration of use. P for trend = .03. B, Time since discontinuation of aspirin and hepatocellular carcinoma risk. Current aspirin use (reference group) was defined as consumption of 2 or more standard-dose (325-mg) aspirin tablets per week on the most recent questionnaire. Among prior aspirin users, time since discontinuation of regular use was defined as nonregular use on the most recent questionnaire but regular aspirin use less than 8 or 8 or more years in the past. P for trend was calculated using continuous elapsed time in months since last regular aspirin use among prior aspirin users. The multivariable-adjusted model was conditioned on age (continuous years), year of questionnaire return and sex (ie, cohort), and was further adjusted for race/ethnicity (white vs nonwhite), body mass index (continuous measure), alcohol intake (0-4.9, 5.0-14.9, ≥15.0 g/d), smoking status (current vs prior vs never), physical activity (<3.0, 3.0-8.9, ≥9 metabolic equivalent task–hours per week), diabetes (yes vs no), hypertension (yes vs no), dyslipidemia (yes vs no), regular multivitamin use (≥2 multivitamin tablets per week vs no), regular use of oral antidiabetic medications (yes vs no), and regular use of statins (yes vs no). All relevant covariates were updated over time. P for trend = .006. HR indicates hazard ratio; error bars, 95% CI.