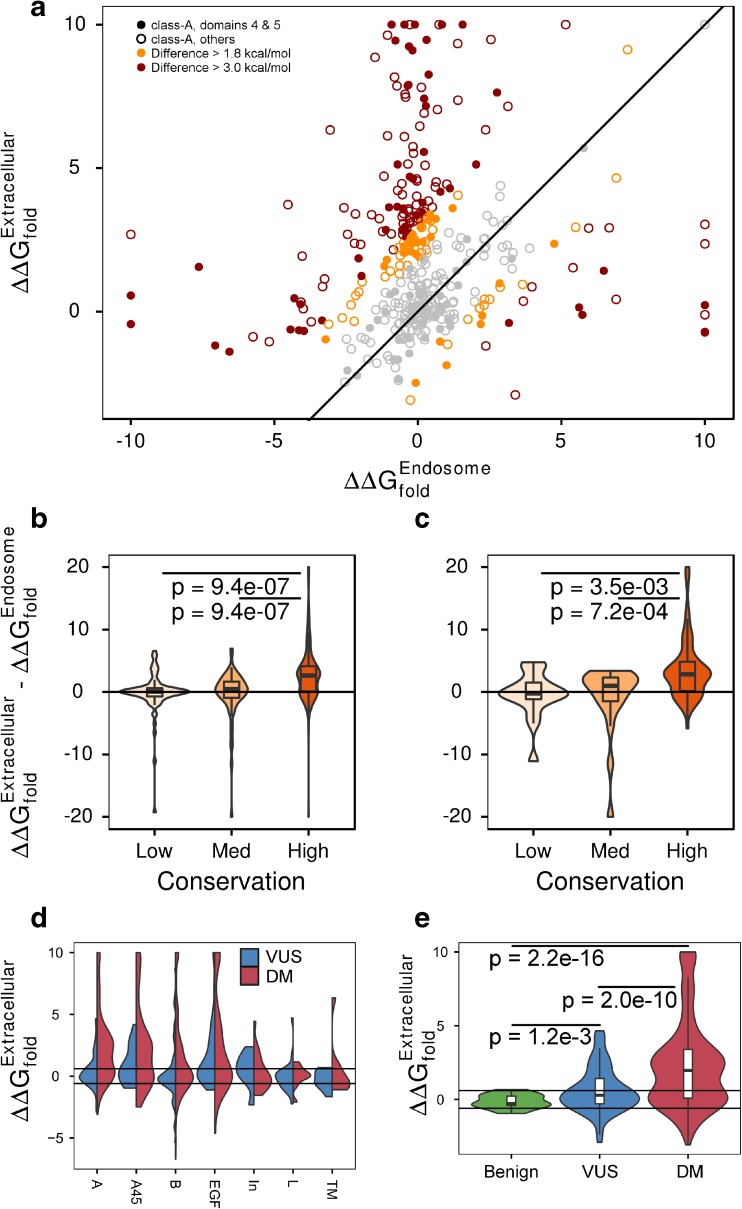
Fig. 3.
LDLR variants have context-specific effects. Each variant may confer significantly different effects on protein stability between endosomal and extracellular conditions. A Each data point represents a different LDLR variant. We evaluated 403 unique missense genomic variants observed in population (ExAC) or disease (ClinVar or HGMD) databases within the class-A domains. Symbols are filled in for the 128 variants from the fourth and fifth class-A domains. The line of equivalence is shown and variants colored gray if they exhibit a difference of less than 1.8 kcal/mol. The 57 (14%) of variants with a difference between 1.8 and 3.0 kcal/mol are colored orange, and the 119 (30%) variants with a difference greater than 3.0 kcal/mol colored red. B Across all class-A domains, there is a significant relationship between residue conservation and the difference in stability between conditions. C This relationship is present within the fourth and fifth class-A domains. D Across LDLR domains, missense variants in the class-A domains have the strongest separation in ΔΔGfold between pathogenic variants and VUS. Horizontal lines mark 0.6 kcal/mol. Pathogenic missense variants in all extracellular domains are more likely to be destabilizing to the native structure compared to VUS. Many VUS in the fourth and fifth class-A and EGF domains are destabilizing. E For our extracellular model of class-A domains, there are strong differences between the distribution of ΔΔGfold among benign, VUS, and pathogenic variants. Not all pathogenic variants destabilize the conformation, but a significant fraction does. A smaller, but still significant proportion of VUS is destabilizing, but no benign variants are destabilizing