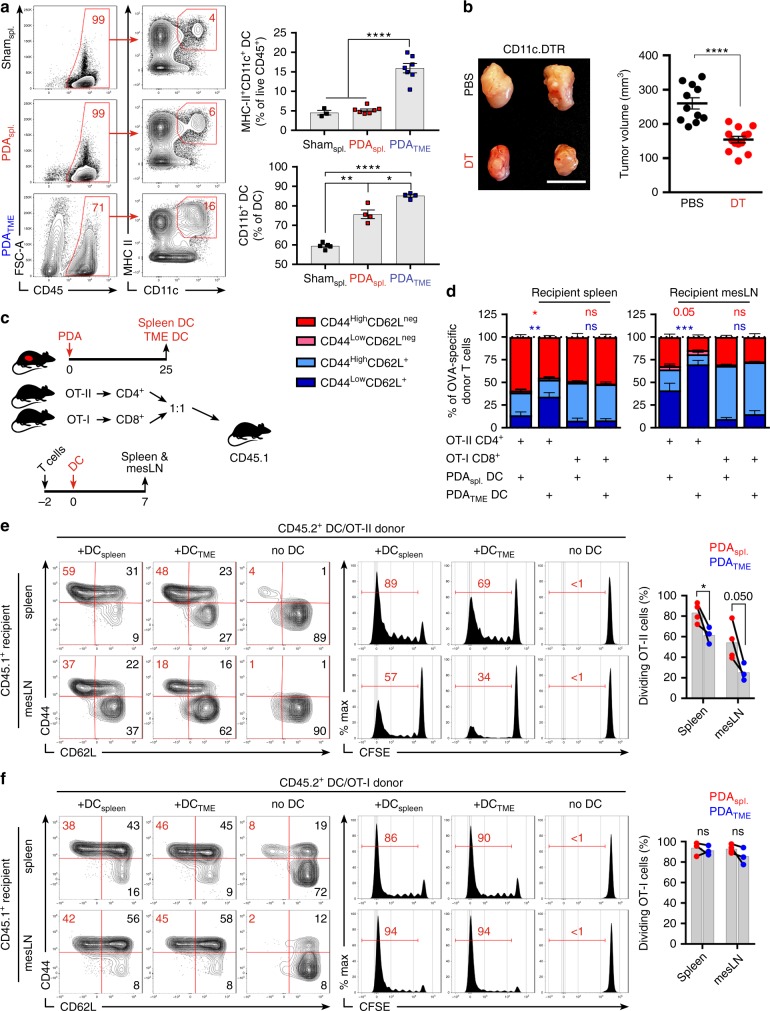
Fig. 1.
Distinct pancreatic ductal adenocarcinoma (PDA)-infiltrating dendritic cell (DC) promote tumor progression and regulate CD4+ T-cell differentiation. a CD45+ cells from the spleen or tumor of orthotopic PDA-bearing mice or from the spleen of control mice were gated using flow cytometry and tested for co-expression of CD11c and MHCII. CD11c+MHCII+ cells were then sub-gated and tested for expression of CD11b. Representative contour plots and quantitative data are shown. This experiment was repeated >5 times. b WT mice were made chimeric using bone marrow from CD11c.DTR mice. Cohorts were challenged with orthotopic PDA 7 weeks later. On day 12, mice began serial treatment with diphtheria toxin (DT) or phosphate-buffered saline (PBS) before sacrifice on day 25. Representative gross images of tumors and quantitative analysis of tumor volume are shown. This experiment had similar results for >5 times (scale bars = 1 µm). c–f PDATME DC were harvested on day 25 from tumor-bearing mice, loaded with either Ova323–339 or Ova257–264 peptide and administered in equal number i.p. to CD45.1 mice that had been transferred i.v. 2 days prior with equal numbers of CFSE-labeled OT-I and OT-II T cells. Parallel experiments were performed using PDAspl. DC. On day 7 after either PDATME or PDAspl DC.Ova administration, spleen and mesenteric lymph nodes (LNs) were harvested and CD45.2+ CD4+ and CD8+ T cells tested for co-expression of CD44 and CD62L and dilution of CFSE. c A schematic of the experimental regimen is depicted. d Quantitative results and e representative flow cytometry data for CD4+ T cells and f CD8+ T cells are shown. This experiment was repeated twice (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, t-test), SEM