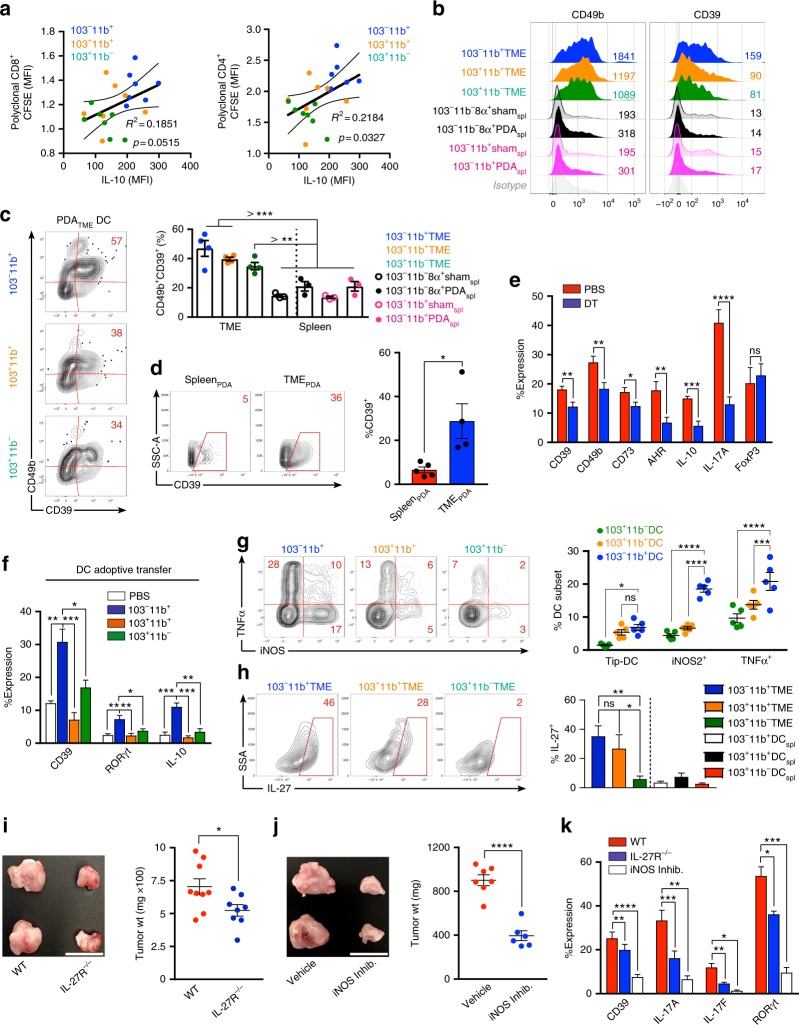
Fig. 5.
Pancreatic ductal adenocarcinoma (PDA)-infiltrating dendritic cell (DC) subsets direct distinct CD4+ T-cell programs in PDA. a CFSE-labeled naive CD8+ or CD4+ polyclonal T cells isolated from the spleens of WT mice were cultured for 96 h on αCD3ε/αCD28-coated plates in the presence of conditioned media from 8-day OT-II CD4+ T cell/PDATME DC subset co-cultures. Polyclonal T cells were assessed by flow cytometry for CFSE dilution. Each individual well from the OT-II/PDATME DC subset co-cultures was used to generate conditioned media for a single well of polyclonal CD8+ and CD4+ T cells. Scatter plots are shown correlating the CFSE dilution of polyclonal T cells to the IL-10 expression of OT-II T cells from the respective OT-II/PDATME DC subset co-culture used to generate conditioned media. Linear regression was used to determine the best-fit line (solid) and 95% confidence intervals (dotted lines); p-values indicate significance of a non-zero slope, determined by an F-test. This experiment was repeated twice. b Ova323–339 peptide-pulsed PDATME DC subsets and splenic DC subsets were co-cultured with Ova-restricted CD4+ T cells at a 1:5 ratio for 5 days. CD4+ T-cell expression of CD49b and CD39 were determined compared with isotype control. Representative histograms with MFIs are shown. This experiment was repeated >3 times (n = 3–6 mice). c Ova323–339 peptide-pulsed PDATME DC subsets and splenic DC subsets were co-cultured with Ova-restricted CD4+ T cells at a 1:5 ratio for 5 days. CD4+ T-cell co-expression of CD39 and CD49b were determined. Representative contour plots (PDATME DC) and quantitative data (PDATME DC, PDASpl. DC, and ShamSpl. DC) are shown. Experiments were repeated >3 times (n = 3–6 mice). d Tumor and spleen were harvested on day 25 from mice bearing orthotopic PDA. Tumor-infiltrating and splenic CD4+ T cells were assessed for expression of CD39. Representative and quantitative data are shown. This experiment was repeated >5 times. e WT mice were made chimeric using bone marrow from CD11c.DTR mice. Animals were challenged with orthotopic PDA 7 weeks later. On day 12, mice began serial treatment with diphtheria toxin (DT) or phosphate-buffered saline (PBS) before sacrifice and tumor harvest on day 25. Intra-tumoral CD4+ T cells were gated on flow cytometry and tested for expression of CD39, CD49b, CD73, AHR, IL-10, IL-17A, and FoxP3. This experiment was repeated >5 times (n = 3–10 mice per group). f KPC-derived tumor cells were orthotopically implanted in pancreata of WT mice admixed with either PBS or each PDATME DC subset previously harvested from other PDA tumors. Tumors were then harvested on day 25 and intra-tumoral CD4+ T cells were gated on flow cytometry and tested for expression of CD39, RORγt, and IL-10. This experiment was repeated twice (n = 5 per group). g PDATME DC subsets were tested for co-expression of TNFα and iNOS. Representative contour plots and quantitative data are shown. This experiment was repeated three times. h PDATME and PDAspl. DC subsets were tested for expression of IL-27 compared with isotype control (data not shown). Representative contour plots and quantitative data are shown. This experiment was repeated >3 times (n = 5). i WT and IL-27R−/− mice were challenged with orthotopic PDA before sacrifice on day 25. Representative pictures of tumors and quantitative data of tumor weight are shown. Each dot represents data from a single mouse. This experiment was repeated three times (Scale bars = 1 µm). j Orthotopic PDA-bearing mice were serially treated with an iNOS inhibitor or vehicle. Tumor volume was measured at 25 days. Representative pictures of tumors and quantitative data of tumor weight are shown. Each dot represents data from a single mouse. This experiment was repeated twice (Scale bars = 1 µm). k WT and IL-27R−/− mice were challenged with orthotopic PDA before sacrifice on day 25. In addition, select cohorts of WT mice were treated with an iNOS inhibitor (n = 5 per group). CD4+ T-cell expression of CD39, IL-17A, IL-17F, and RORγt were determined. This experiment was repeated twice (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, t-test), SEM