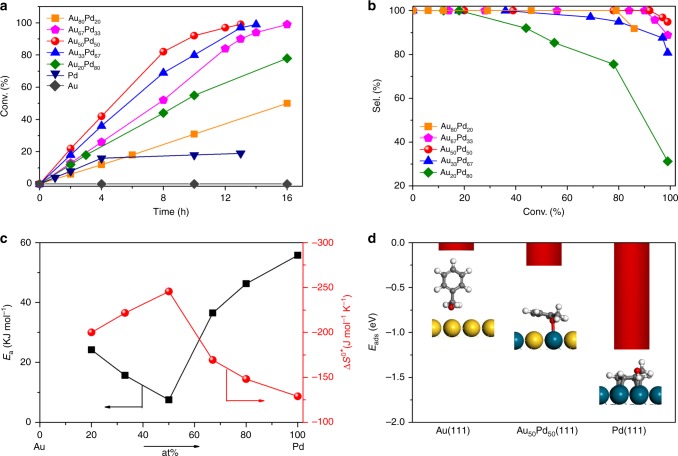
Fig. 5.
Kinetics analysis and DFT calculations. a Benzyl alcohol conversion (Conv.) as a function of time with the following reaction conditions over monometallic Au, Pd and AuPd alloy nanocatalysts. b Compile of the selectivity (Sel.) to benzyl aldehyde as a function of benzyl alcohol conversion over AuPd alloy nanocatalysts. c Relationship between the activation energy (Ea) and entropy of activation (ΔS0*) for the oxidation of benzyl alcohol to benzaldehyde on the AuPd alloys as a function of concentration. d The most favourite configuration and the adsorption energy (Eads) of benzyl alcohol over Au(111), Pd(111) and Au50Pd50(111) by DFT calculations. The reaction conditions were: 32 mg of catalyst; 5.0 mmol of substrate; 10 mL of water; 90 °C; and in the presence of oxygen by an O2 balloon under atmospheric pressure