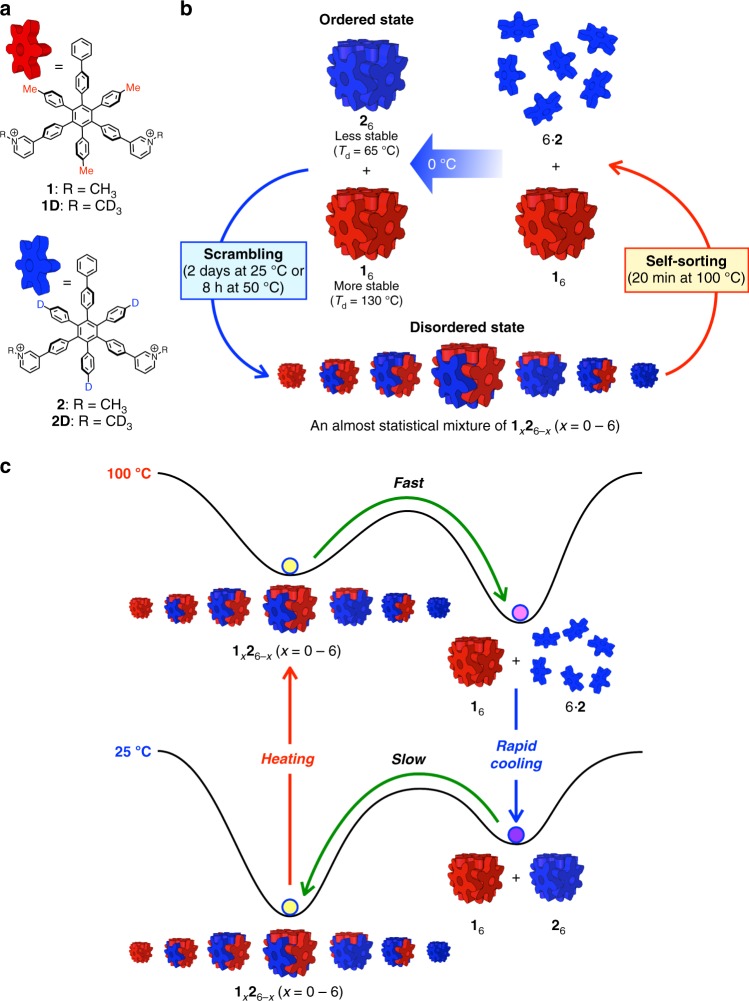
Fig. 1.
A temperature-controlled cycle of transitions between ordered and disordered states in a mixture of nanocubes (16 and 26). a The chemical structures of GSAs used in this research. b A temperature-controlled cycle of the conversion between the ordered and disordered states. An almost statistical mixture of the nanocubes (disordered state) was produced at 25 or 50 °C, while a mixture of two kinds of nanocubes composed of single GSAs was preferentially produced by heating at 100 °C followed by rapid cooling at 0 °C. There are positional isomers for 1x26−x (x = 2–4). c A schematic representation of change in the energy landscape of a mixture of two kinds of GSAs, 1 and 2, in response to the temperature. An almost statistical mixture of nanocubes 1x26–x (x = 0–6) is thermodynamically most stable at 25 °C, while a mixture of 16 and monomer 2 is thermodynamically most stable at 100 °C because decomposition temperatures of the nanocubes composed of more GSAs 2 are lower than 100 °C