Abstract
The primary Brazilian malaria vector, Nyssorhynchus darlingi (formerly Anopheles darlingi), ranges from 0°S–23°S across three biomes (Amazonia, Cerrado, Mata Atlântica). Rising temperatures will increase mosquito developmental rates, and models predict future malaria transmission by Ny. darlingi in Brazil will shift southward. We reared F1 Ny. darlingi (progeny of field-collected females from 4 state populations across Brazil) at three temperatures (20, 24, 28 °C) and measured key life-history traits. Our results reveal geographic variation due to both genetic differences among localities and plastic responses to temperature differences. Temperature significantly altered all traits: faster larval development, shorter adult life and overall lifespan, and smaller body sizes were seen at 28 °C versus 20 °C. Low-latitude Amazonia mosquitoes had the fastest larval development at all temperatures, but at 28 °C, average development rate of high-latitude Mata Atlântica mosquitoes was accelerated and equivalent to low-latitude Amazonia. Body size of adult mosquitoes from the Mata Atlântica remained larger at all temperatures. We detected genetic variation in the plastic responses among mosquitoes from different localities, with implications for malaria transmission under climate change. Faster development combined with larger body size, without a tradeoff in adult longevity, suggests vectorial capacities of some Mata Atlântica populations may significantly increase under warming climates.
Introduction
Although the average incidence of malaria decreased by 22% across the South American continent between 2010–2016, nearly all countries in the Amazonia biome experienced an increase, especially Venezuela in 20171,2. In Brazil, Amazonia has long been a hotbed of malaria transmission2–4 and remains a complex region for the implementation of successful control interventions5,6. In particular, recent studies have highlighted the impact of deforestation7, high prevalence of asymptomatic cases, difficulties in surveillance and medical treatment, and human migration8 on the maintenance of malaria in this region.
An essential but under-investigated driver of malaria is the vector Nyssorhynchus darlingi (formerly known as Anopheles darlingi)9, distributed throughout much of Latin America. This species readily colonizes habitats with diverse ecological characteristics10,11, and in Brazil is widely distributed across three biomes, Amazonia, Cerrado and Mata Atlântica. There is evidence of genetic differentiation within and among Ny. darlingi populations across these biomes12, as well as within the Brazilian13 and Peruvian Amazonia biome14. Such differentiation suggests that a combination of genetic variation and phenotypic plasticity (the ability of individual genotypes to produce different phenotypes in different environments) may enable adaptation to a range of ecological conditions. Mosquito development can be affected by many environmental factors15–18 that directly and indirectly affect both vectorial capacity and malaria transmission16–20. Analyses of latitudinal clines often reveal that intraspecific trait variation is associated with environmental conditions15,21. Many insects (but not all22) such as the fruit fly (Drosophila melanogaster21), cabbage beetle (Colaphellus bowringi23), and mosquito (Culex coronator24) show increasing body size with increasing latitude. Such patterns of geographic variation can result from phenotypic plasticity, genetic adaptation, or a combination of the two25.
Phenotypic plasticity is a property of the genotype, and reaction norms can be studied to identify environmental, genetic, and genotype-by-environment effects26,27. Plasticity has been shown to influence the variability in host preference28 and ability to colonize new ecological niches of the African malaria vector Anopheles gambiae s.s.29. However, the study of phenotypic plasticity in life history traits of Ny. darlingi has been limited to wing size and shape30,31 and gonotrophic concordance32. In contrast to field collections, data from laboratory-reared populations can be used to disentangle genetic and plastic effects33.
Temperature is well documented to influence body size and development time in insects34, and all life stages of mosquito populations35–37. Temperatures are projected to rise 1–4 °C over the next 100 years as a result of climate change38. Changes in temperature can influence malaria distribution because female biting rate, adult mortality rate, parasite development rate, vector competence and, accordingly, vectorial capacity are temperature-dependent parameters17. Currently, malaria transmission has been recorded at average monthly temperatures up to 32 °C in Brazil39. Using data from An. gambiae, An. stephensi and An. pseudopunctipennis, models predict that mosquito populations can remain stable up to 33 °C40. In contrast, models and experiments found that the optimal temperature for Plasmodium spp. is around 25 °C17,41.
It is unclear how future climate change will affect the future distribution of malaria, as such change impacts every component of transmission, from vector dynamics to parasite development rate37,42,43. Current Ny. darlingi distributions and climate estimates have been used for projection models, and these forecast range expansion of this vector and increased intensity of malaria in southern Brazil10,11, due to the shifts in climate expected for Brazil (Fig. 1).
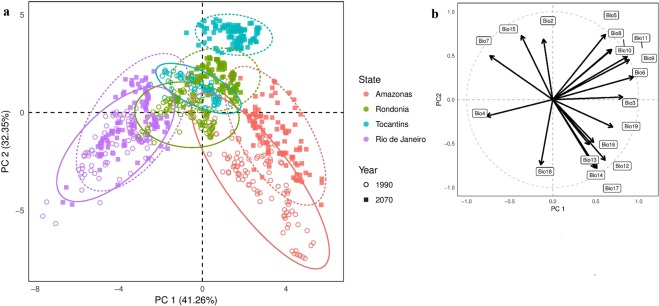
Figure 1.
PCA of past (1960–1990) and future (2070) climates in each state (a) extracted based on 100 random GPS localities within each state and all 19 Bioclim variables at 30 arc second resolution, (b) correlation plot of 19 bioclimatic variables. Both past (1960–1990) and future (2070) climate data are from WorldClim82. The states of Amazonas and Rio de Janeiro have the most disparate climates now and in the future. Rondônia has little overlap with Amazonas despite being in the same biome. Rondônia and Tocantins, states at the same latitude but in different biomes, overlap some in current climate, but not in the future. In an extreme case, Tocantins shows no overlap between current and future climates. See additional details in supplement and Supplementary Table S5 for variable descriptions.
Life history trait estimates can be useful in directing control strategies44,45 and malaria elimination will require combinations of interventions at different life stages46,47 with frequent monitoring and evaluation48. In general, mosquito vector research has focused on adult components, often overlooking features of larval development important for the assessment of vectorial capacity. The latter integrates different aspects of mosquito and parasite life histories, e.g., daily female survival rate, human blood feeding rate, vector density, and the extrinsic incubation period19. Larger mosquitoes with increased survival and fecundity49,50 have been associated with increased food quantity16,18,51, reduced larval density and competition16, and lower rearing temperatures15,17,51,52. Laboratory experiments using colonies of An. gambiae and An. stephensi determined that increasing mean ambient and diurnal temperature reduced overall vectorial capacity through a reduction in parasite viability and mosquito survival rate at temperatures exceeding 30 °C20. Recent modeling40, field studies53 and laboratory rearing experiments16,18,20 measured life history traits under a range of environmental conditions and found that vectorial capacity values were highly variable. This suggests possible roles for genetic differentiation or phenotypic plasticity of vectors affecting rates of disease transmission.
Theoretical models have shown that populations with high phenotypic and genetic variability are expected to have broader niches, increased population growth rate, and increased resilience to environmental change54. Comparative study of phenotypic plasticity of life history traits can provide significant insight into the ecological genetics of widely distributed species. In the present study, we investigate how differences in rearing temperature and population affect life history traits of Ny. darlingi from three Brazilian biomes. Our experimental design allows us to partition phenotypic variation among populations into plastic and genetic components as well as their interaction (i.e., whether there is genetic variation for plasticity). Studying populations from different Brazilian states across the distribution of Ny. darlingi (Fig. 2) under a range of temperature treatments will provide baseline estimates of genetic variation and phenotypic plasticity that can inform future modeling efforts, tailor vector control efforts, and guide the design of novel anti-malarial strategies42.
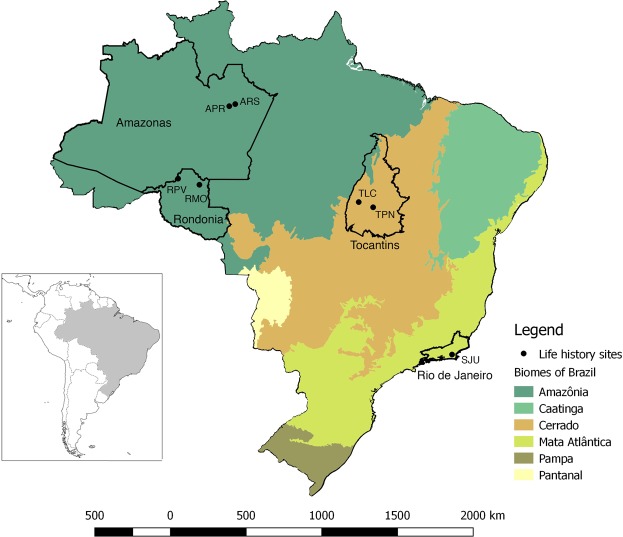
Figure 2.
Map of Ny. darlingi collection sites across Brazil. Biome distributions are from publicly available maps83.
Results
Laboratory rearing and survivorship
The initial dataset consisted of 3,430 individuals; 778 died before adult emergence. Proportional tests were conducted, and the interaction of temperature and state marginally affected larval survival to adulthood (M2 = 10.9, 6 d.f., p = 0.09) (Supplementary Table S1). In general, survivorship to adulthood in the laboratory decreased with increasing latitude and temperature, but the significant interaction of temperature and state indicates that the magnitude of temperature-related reduction is dependent on state (Supplementary Tables S2–S3). Except for Amazonas state, mosquitoes from all states had significantly higher mortality at 28 °C: populations of mosquitoes from Rondônia and Tocantins had ~16% reduced survival, whereas for Rio de Janeiro, a much steeper reduction of nearly 60% was observed (Supplementary Fig. S1 and Table S4). All further analyses were conducted on the 2,652 individuals that survived to adulthood (Table 1).
Table 1.
Characteristics of seven collection sites in Brazil and sample size of Ny. darlingi tested in laboratory for life history traits at each of three temperatures (total n = 2,652).
| Biome | State | Locality | Latitude | Average mean temp (min, max)* | No. mosquitos at each temp. | ||
|---|---|---|---|---|---|---|---|
| 20 °C | 24 °C | 28 °C | |||||
| Amazon | Amazonas | ARS | −2.864 | 27.6 (26.9, 28.6)a | 134 | 135 | 130 |
| APR | −3.028 | 27.7 (26.7, 29.2)a | 198 | 198 | 179 | ||
| Rondônia | RPV | −8.742 | 25.7 (23.2, 26.6)b | 181 | 184 | 141 | |
| RMO | −9.223 | 25.6 (22.8, 26.5)b | 192 | 197 | 160 | ||
| Cerrado | Tocantins | TLC | −10.7 | 28.4 (23.3, 35.4)a | 92 | 84 | 71 |
| TPN | −10.796 | 27.5 (26.2, 28.6)a | 32 | 35 | 24 | ||
| Mata Atlantica | Rio de Janeiro | SJU | −22.611 | 21.2 (20.5, 22.3)c | 129 | 123 | 33 |
Variation in life history due to temperature and state
Analysis of Variance (ANOVA) showed significant variation in adult longevity, larval development and wing length due to temperature treatment and state of origin (Table 2). Variation in traits among states within temperature treatments is evidence of significant additive genetic variation among states (Figs 3 and S4). Significant differences within states across temperature treatments is evidence of phenotypic plasticity (Figs 3 and S4). The significant interaction between temperature and state (Table 2) indicates that Ny. darlingi populations from these regions also have genetic differences in plastic responses of larval development, adult longevity and wing length (i.e., differences in slopes in Fig. 3).
Table 2.
ANOVA of life history traits by state and temperature.
| Trait | Source | d.f. | MS | F | p |
|---|---|---|---|---|---|
| Larvae development | State | 2 | 2349.67 | 530.91 | <2.2e-16 |
| Temperature | 3 | 444.26 | 100.38 | <2.2e-16 | |
| S × T | 6 | 38.1 | 8.61 | 4.16e-08 | |
| Adult longevity | State | 2 | 505.15 | 883.66 | <2.2e-16 |
| Temperature | 3 | 11.44 | 20.01 | 6.66e-10 | |
| S × T | 6 | 9.53 | 16.67 | 4.44e-15 | |
| Wing length | State | 2 | 6.4327 | 459.61 | <2.2e-16 |
| Temperature | 3 | 0.4964 | 35.46 | 9.77e-15 | |
| S × T | 6 | 0.1388 | 9.92 | 2.52e-09 |
Significant p-values are in bold, d.f. - degrees of freedom; MS - mean squares.
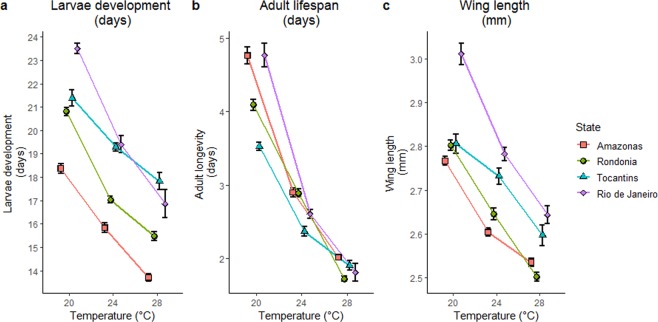
Figure 3.
Average duration of larvae development (days, a) average adult life (days, b) and wing length (mm, c) with standard error bars graphed by state. See Supplementary Fig. S4 for significance of differences among states.
Increasing temperature significantly reduced the average length of larval development time within each state (Figs 3a and S4). At the latitude extremes, Amazonas (Amazon) and Rio de Janeiro (Mata Atlântica) larval development differed by 4 to 6 days at all temperatures (Supplementary Fig. S4). Average larval development was distinct by latitude in the low temperature treatment (20 °C), but this distinction was lost in the higher temperature treatments (Supplementary Fig. S4). Within Amazonia, larval development of populations from Amazonas and Rondônia states differed by 2 or 3 days at all temperatures (Fig. 3a and Supplementary Table S6). Duration of pupal stage (Supplementary Fig. S2 and Table S7) and total time to emergence (larval and pupal stages) (Supplementary Fig. S3 and Table S8) showed similar trends.
Average unfed adult life span was significantly reduced as temperature increased within each state (Figs 3b and S4). At the latitude extremes, Amazonas (Amazon) and Rio de Janeiro (Mata Atlântica) had equivalent adult life spans at both 20 and 28 °C (Supplementary Fig. S4). Within Amazonia, adult life spans of populations from Amazonas and Rondônia states differed only at 20 °C and 28 °C (Fig. 3b and Supplementary Table S9).
Average wing length was significantly reduced with increasing temperature within each state (Fig. 3c, Table 2, Supplementary Fig. S4 and Table S10). Amazonia (Amazonas, Rondônia) populations and the Mata Atlântica (Rio de Janeiro) population were distinct at all temperatures, with average differences between 0.1 and 0.2 mm (Supplementary Fig. S4).
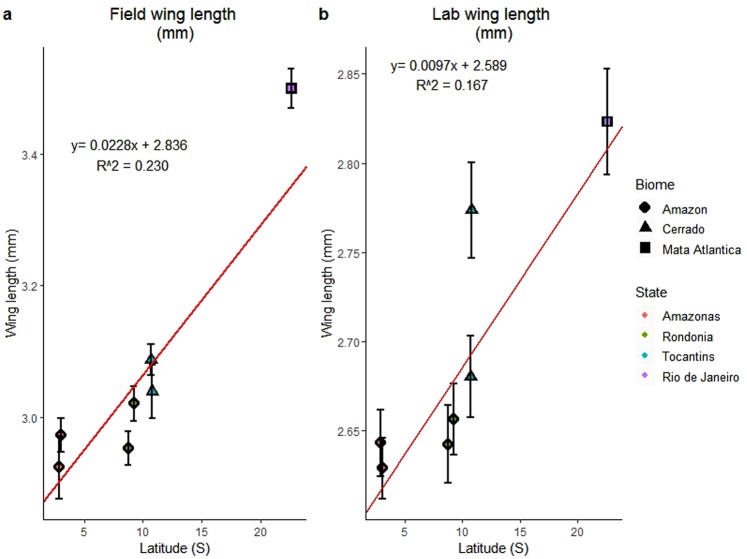
Wing length increases with latitude in field and lab populations
Field-collected mosquitoes showed a Bergmann cline, where wing lengths increased with increasing latitude, and Rio de Janeiro state mosquitoes were the largest (Fig. 4a). We also find evidence of a genetic basis for the cline, with lab-reared mosquitoes also showing a positive relationship between wing lengths and latitude of origin (Fig. 4b). There was a greater difference in size of F1 lab-reared adults between the Tocantins state sites compared to the field-collected females from those sites (Fig. 4).
Figure 4.
Evidence for Bergmann’s Rule. (a) Means (±SE) of population wing length measurements of field-collected mosquitoes are positively associated with latitude. (b) Means (±SE) of populations across temperature treatments for lab-reared, adult mosquitoes associated with latitude of origin, showing evidence of a genetic basis for Bergman’s cline in Ny. darlingi. Regression lines and equations correspond to OLS analysis. Note: scales of (a) and (b) y-axes differ.
Total lifespan decreases with increasing temperature
The Kaplan-Meier survival curves indicated decreased lifespan within and across states as temperature increases (Fig. 5 and Table 3). Cox regression showed that increasing temperature within populations (z = 26.6, p < 2e-16) led to a significant decrease in survival, while a significant interaction of temperature with increasing latitude (z = 3.7, p < 2e-4) indicated that the 4 states differ in their overall survivorship curves.
Figure 5.
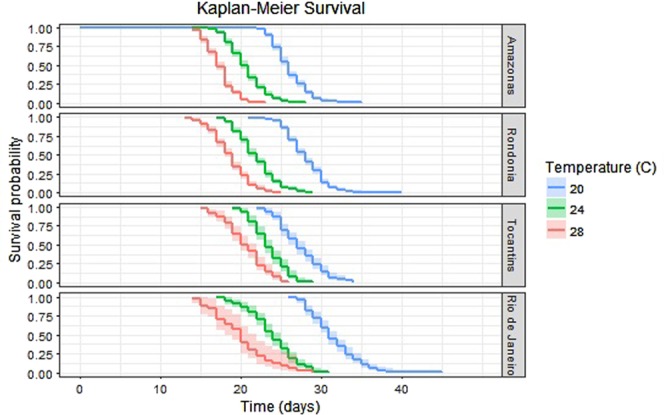
Kaplan Meier survival curves of mosquitoes that eclosed to adult (n = 2,652). These visualize total lifespan probabilities of mosquitoes within each state at each temperature treatment over time. There is a significant interaction between state and temperature, i.e., there are significant differences in the shapes of the survival curves.
Table 3.
Probability of survival (±standard error) of the 2,652 mosquitoes that eclosed at 4 time points (15, 20, 25, 30 days post hatch) by temperature and state.
| 20 °C | 24 °C | 28 °C | 20 °C | 24 °C | 28 °C | ||
|---|---|---|---|---|---|---|---|
| Amazonas state | Rondônia state | ||||||
| Probability survival (±standard error) | Day 15 | 0.99 ± 0.003 | 0.86 ± 0.019 | 0.49 ± 0.028 | 1.0 ± 0.00 | 0.95 ± 0.011 | 0.70 ± 0.026 |
| Day 20 | 0.59 ± 0.026 | 0.09 ± 0.016 | 0 | 0.94 ± 0.011 | 0.25 ± 0.022 | 0.063 ± 0.014 | |
| Day 25 | 0.03 ± 0.009 | 0 | 0 | 0.23 ± 0.022 | 0.008 ± 0.004 | 0 | |
| Day 30 | 0 | 0 | 0 | 0.005 ± 0.004 | 0 | 0 | |
| Tocantins state | Rio de Janeiro state | ||||||
| Probability survival (±standard error) | Day 15 | 1.0 ± 0.00 | 1.0 ± 0.00 | 0.88 ± 0.033 | 1.0 ± 0.00 | 0.98 ± 0.011 | 0.69 ± 0.08 |
| Day 20 | 0.92 ± 0.024 | 0.59 ± 0.045 | 0.25 ± 0.045 | 0.99 ± 0.007 | 0.63 ± 0.044 | 0.21 ± 0.071 | |
| Day 25 | 0.28 ± 0.04 | 0.017 ± 0.012 | 0 | 0.69 ± 0.041 | 0.07 ± 0.024 | 0.03 ± 0.029 | |
| Day 30 | 0 | 0 | 0 | 0.05 ± 0.019 | 0 | 0 | |
Discussion
Most laboratory rearing studies of Anophelinae malaria vectors have relied on specimens from established colonies16,18,37,50,55–58, and thus, the range of biomes and latitudes of the populations in our study had not previously been examined29,36. Although studies using colony-reared specimens are invaluable, studying the F1 generation of wild-captured adult females has provided us with new insights into regional differences, including differences in both the mean values of traits and their plasticities.
Our findings indicate that geographic variation in Ny. darlingi life history traits results from both genetic differences among localities as well as plastic responses to differences in temperature. Mosquitoes from higher latitudes are genetically distinct (larger with longer lives) at 20 °C, but these differences in size and adult lifespan are diminished as temperatures rise to 24° and 28 °C. Our laboratory results verify that the wing size-latitude cline seen for field-collected populations is genetically based (Fig. 4). Genetic variation in life history traits indicates that Ny. darlingi could adapt to novel environmental conditions and may at least partly explain its success as the predominant South American malaria vector, colonizing many anthropogenic habitat types59,60. Further, our results demonstrate that there is genetic variation in the plastic responses among mosquito populations derived from different localities: the reaction norms (shapes of the curves) depicting responses to temperature vary among populations of different regions (Fig. 3).
Larval rearing studies have previously shown how rearing conditions, such as reduced nutrition and increased competition, reduced adult fitness16,57,61. In agreement with other mosquito life history studies16,56,62, increasing temperature reduced the average duration of larval development, adult life span, body size, and overall survival. The lowest latitude population in Amazonas state had the fastest larval development at every temperature. Mosquitoes from the population with the coolest climate, Rio de Janeiro state, developed nearly 5 days slower at the lowest temperature tested (20 °C) than those from Amazonas. However, the average unfed adult lifespan of Ny. darlingi from these two states was the same (~5 days). A study of colony An. gambiae found that longer development at a lower temperature resulted in larger mosquitoes, but adult longevity was the same across three larvae rearing temperatures61. We also found a positive association between development time and body size, but, in contrast, we found large differences in adult longevity across temperatures (Fig. 3b).
Research on conditions, such as temperature, that affect vector development has become a priority recently, because of the impact on malaria-relevant traits, e.g., vector competence63 or insecticide resistance55. Here, we show that, as expected, larval development, adult size and lifespan of Ny. darlingi are significantly affected by temperature. Most strikingly, we demonstrated that the nature of the plastic responses to temperature varies among regions: populations from warmer climates have flatter reaction norms in response to temperature, especially in relation to larval development (Fig. 3a). We found that difference in larval development (Fig. 3a) and adult size (Fig. 3c) are maintained at higher temperatures. Taken together, these results suggest that significant population-level differences in vectorial capacity will emerge as climate continues to warm and malaria moves south.
However, we also need to consider the significant mortality experienced by high-latitude mosquitoes. We are unsure why mosquitoes from Tocantins and Rio de Janeiro states should have lower overall survival in the laboratory – perhaps due to differences in their ability to tolerate laboratory humidity or constant temperature. Even if we set their overall survival rates aside, there is still a marked increase in mortality in these populations at 28 °C. Thus, reduced survival at higher temperatures may offset the life history advantages noted above that surviving individuals from these populations have gained. Of note is the broad spread of survival values among families within the Rio de Janeiro population - to the extent that this variation reflects genetic differences, the Rio de Janeiro population may still contain significant raw material for adaptation to higher temperatures.
Possible study limitations
Our findings provide baseline information on the general relationship of temperature to larval development and adult traits, though there are some limitations. First, our study of F1 progeny could not exclude the impact of maternal effects, such as maternal fitness or nutritional content of blood meal prior to egg laying. There is no reason to believe, however, that such effects would be biased to be stronger in some localities than others. Second, there has been an increase in deforestation7 and urban development within the biomes that has altered the landscape at a local level, obscuring biome-level comparisons to some degree by introducing greater environment heterogeneity. Additionally, diurnally fluctuating temperatures and feeding of adults may produce results that are more useful for prediction of malaria transmission. In a previous study, fluctuating temperature, compared to constant temperatures, increased the rate of parasite development at lower temperatures, with the opposite effect observed at higher temperatures64. Meanwhile, models investigating temperature fluctuations on mosquito population dynamics found that total adult abundance might be reduced compared to constant temperatures65.
All localities were sampled just once in 2016 during lower precipitation months (Supplementary Table S1). The adult longevity observed in our study deviates from natural adult longevity that would involve regular adult feeding. For example, in An. gambiae, a colony-sourced population that was sugar-fed lived an average of 22 days66 compared with a field-sourced population that lived an average of 2 days when provided only water as adults67. Such differences between lab vs. field adult longevity preclude us from using lab longevity for vectorial capacity calculations16.
Recent colony establishment of Ny. darlingi will facilitate additional studies on mosquito development, with research already underway on vector competence68. Future laboratory work should be continued on field populations to investigate regional variation of blood feeding, fecundity, and vectorial capacity. Characterization of field populations will ultimately provide local information that can be updated to monitor the changing vector landscape and the effectiveness of disease abatement strategies.
Conclusion
Projection models suggest that, in addition to the expected temperature increases38, climate differences among regions will become more pronounced in the future (Fig. 1). Our results indicate that higher latitude populations of Ny. darlingi may be able to tolerate a larger range of temperatures than they currently experience. This supports models suggesting that climate change is expected to increase the intensity of malaria cases in South America with increased abundance of Ny. darlingi in the south10.
Populations of Ny. darlingi vary not just for life history trait means, but also for how those traits respond to temperature variation. Importantly, this suggests that the relatively slow development at lower temperatures of higher latitude populations (such as those in the Mata Atlântica), may become much more rapid as temperatures increase. Adult longevities are not different among populations at high temperatures (Fig. 3b), but higher latitude populations stay larger at high temperatures.
The combination of faster development, and larger body size, without a tradeoff in adult longevity could lead to higher vectorial capacities of these southern populations. While the Rio de Janeiro population, as a whole, has much lower survival to adulthood in the lab at 28 °C, some families have much higher survival. If lower survival is not a factor in the field, or if the variation for survival in this population has a genetic basis, our results suggest that the Rio de Janeiro population may adapt to higher temperatures. Coupled with its life history characteristics at high temperatures, this could lead to significant increases in vectorial capacity in warming climates.
Methods
Mosquito collection
Mosquitoes were collected across four states representing 3 biomes: Amazonas, Rondônia (Amazonia biome), Tocantins (Cerrado biome) and Rio de Janeiro (Mata Atlântica biome) (Fig. 2). Collection-site pairs within a state were chosen using the following criteria: (i) proximity to forest edge and larval habitat suitable for Ny. darlingi; (ii) proximity to human dwellings; (iii) same latitude (±1°) and between 100–200 km apart; and (iv) no significant geographic barrier (river, mountains) between within-state pairs. However, for Rio de Janeiro, only one site yielded mosquitoes; repeated collection attempts failed at a second site where Ny. darlingi was known to occur12. Mosquitoes were collected in the evening for 5 hours (17:00–22:00) using barrier screens as described in Moreno et al.69. Blood-fed female mosquitoes from barrier screens were morphologically identified as Ny. darlingi70 and maintained individually in a humid box and provided ad libitum sucrose solution during transport to the laboratory (Laboratório de Entomologia da Faculdade de Saúde Pública, Universidade de São Paulo). Collection and egg laying data are provided (Supplementary Table S11).
Biome descriptions
The Amazonia biome comprises ~61% of Brazil and is characterized by year-round high temperatures, high humidity, and a complex forest-river ecosystem. The Cerrado covers ~21% of Brazil, stretching across the central, semi-humid grasslands with perennial warm temperatures. The somewhat fragmented, mostly coastal, Mata Atlântica supports a cooler, humid climate and comprises mainly dense second growth forests with multilevel canopies (Fig. 2)3,60,71,72.
Egg oviposition
Mosquitoes were allowed sufficient time for digestion and egg development before individual preparation for egg laying, either within 24 hours of arriving at the laboratory or 36–48 hours post blood meal. Each mosquito was briefly anesthetized with ethyl acetate vapor, the left wing removed, and then each mosquito was placed in an individually labeled oviposition cup containing distilled water for 36–48 hrs. at room temperature (26 °C ±2). Fine mesh was laid on top of the egg cups to minimize disturbance and prevent individual mosquitoes from escaping or disturbing other egg cups. Larvae from each cup constituted an individual maternal family and were not pooled with other larvae, and populations were randomized to minimize potential maternal effects.
Mosquito rearing
All larvae, pupae and adults were maintained in temperature and light controlled chambers (12:12 light:dark cycle). Environmental chambers were set to 20 °C, 24 °C, 28 °C ± 1 °C. The temperatures used were selected to reflect the natural temperature range of Ny. darlingi across sites73 and to avoid laboratory rearing temperature extremes52. Data loggers (iButtons; iButton Link, LLC, Whitewater, WI, USA) were placed in all chambers to record and monitor temperature hourly to ensure a constant environment.
Larvae hatched within 1–2 days of oviposition. Upon hatching, the first 45 larvae obtained per egg cup were reared, 15 at each of three different temperatures (20 °C, 24 °C, 28 °C). Larvae were randomly assigned to temperatures for rearing in groups of 5. Larvae were reared in 6-well plates, each well containing 3 mL deionized water and 5 larvae, and fed 2 mg of food daily comprising a finely ground mixture (by weight) of Tetramin Tropical flakes (28%), Bettamin Tropical flakes (28%), Tetraveggie Spirulina (27%), Bettamin pellets (16.95%) and bee pollen (0.05%). Any larval deaths were recorded at the same time daily. Larvae were transferred to a clean 6-well plate with fresh water every other day after the 4th day post-hatching. Pupae were collected daily and transferred into individual rearing vials containing 3 mL of deionized water and allowed to eclose. After emergence, adult mosquitoes were transferred to individual rearing vials containing a moistened cotton ball to provide an environment that was consistently between 70–80% relative humidity. Vials were capped with a water-moistened cotton ball to provide additional water; no food was provided. Mortality of pupae and adults was recorded daily.
Wing measurements for body size estimation
The left wing, collected at either egg laying (field) or natural death (lab reared), was mounted on a glass slide with clear-drying glue. In instances where the left wing was damaged, the right wing was used. Wing images were collected using an Olympus X-70 microscope with bright field light at 10x. Wing length was measured, using the digital images, from the alula to the distal end minus the fringe scales to the nearest hundredth millimeter (ImageJ)74. Insect wing length measurements allow for a rapid estimate of body size due to strong correlations between wing length and indicators of adult size (adult dry weight75, pupal mass76).
Statistical analysis
All statistical analysis was conducted in R v3.3.377. To evaluate the significance of among-population differences on survival to adulthood (eclosion), proportional tests were conducted with two-way (Chi square) or three-way interactions (Cochran-Mantel-Haenszel)77. Survivorships (proportions surviving to eclosion) were compared among temperatures and states using a logistic regression78 with a binomial family and a logit link function with temperature and state as fixed effects. Genetic variation and phenotypic plasticity were assessed with ANOVA78, using temperature and state as fixed effects with maternal family as a random effect. Generalized linear mixed models (GLMM), using Penalized Quasi-Likelihood, of family means were created to assess the influence of temperature and state on the life history traits79. Sex was not a significant factor and was dropped from the final models. Survival analyses were performed for each state across the three temperatures using Kaplan-Meier survival analysis80,81. Differences between state and temperature results were compared using a Cox Regression80.
In order to test for the presence of a Bergmann’s cline, linear regression models were constructed to compare wing lengths of field-collected specimens over increasing latitude. To evaluate whether there was a genetic basis to this relationship, the same models were run on laboratory-reared specimens (means across states and temperature treatments).
Supplementary information
Acknowledgements
We extend our sincere thanks to Denise C. Sant’Ana, Eduardo S. Bergo, and Leonardo Chaves for their assistance in field collections and to Caio Morreira for laboratory rearing assistance. We thank Sara Bickersmith for her help with logistics. We appreciate the support of the local communities and health workers of Juturnaiba, Rio de Janeiro; Lagoa da Confusão, and Porto Nacional, Tocantins; Machadinho d’Oeste, and Porto Velho, Rondônia; Ramal Novo Horizonte, and Brasilierinho, Manaus, Amazonas. We are grateful to the Coleção Entomológica de Referência and Faculdade de Saúde Pública, Universidade de São Paulo for laboratory facilities. The research leading to these results was funded by NIH-NIAID (1R01AI110112) to J.E.C. and M.A.M.S. The Boren Awards and the Biodefense and Emerging Infectious Disease training fellowship grant (T32AI05532901) provided partial funding support for V.M.C.
Author Contributions
J.E.C., M.A.M.S., C.D.S. and W.D.L. conceived the initial experimental design and obtained grant funding. V.M.C. and M.A.M.S. conducted field work with V.M.C. conducting all laboratory rearing; analysis was done by V.M.C. with guidance from C.D.S. and T.E.M.; V.M.C., J.E.C, C.D.S. and T.E.M wrote the manuscript with contributions from M.A.M.S. and W.D.L.
Data Availability
All relevant data are within the paper and its Supporting Information Files.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
V. M. Chu, Email: vchu@albany.edu
J. E. Conn, Email: jan.conn@health.ny.gov
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41651-x.
References
- 1.Pan American Health Organization & Report. Report on the situation of Malaria in the Americas. (2016).
- 2.World Health Organization. World Malaria Report 2017 (2017).
- 3.de Pina-Costa A, et al. Malaria in Brazil: What happens outside the Amazonian endemic region. Mem. Inst. Oswaldo Cruz. 2014;109:618–633. doi: 10.1590/0074-0276140228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz LR, Spangenberg T, Lacerda MVG, Wells TNC. Malaria in South America: a drug discovery perspective. Malar. J. 2013;12:168. doi: 10.1186/1475-2875-12-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Castro MC, Monte-Mór RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proc. Natl. Acad. Sci. USA. 2006;103:2452–7. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Castro MC, Sawyer DO, Singer BH. Spatial patterns of malaria in the Amazon: Implications for surveillance and targeted interventions. Heal. Place. 2007;13:368–380. doi: 10.1016/j.healthplace.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Chaves LSM, Conn JE, López RVM, Sallum MAM. Abundance of impacted forest patches less than 5 km2 is a key driver of the incidence of malaria in Amazonian Brazil. Sci. Rep. 2018;8:7077. doi: 10.1038/s41598-018-25344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira MU, Castro MC. Challenges for malaria elimination in Brazil. Malar. J. 2016;15:1–18. doi: 10.1186/s12936-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster PG, et al. Phylogeny of Anophelinae using mitochondrial protein coding genes. R. Soc. Open Sci. 2017;4:170758. doi: 10.1098/rsos.170758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laporta GZ, et al. Malaria vectors in South America: current and future scenarios. Parasit. Vectors. 2015;8:426. doi: 10.1186/s13071-015-1038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alimi TO, et al. Predicting potential ranges of primary malaria vectors and malaria in northern South America based on projected changes in climate, land cover and human population. Parasites and Vectors. 2015;8:1–16. doi: 10.1186/s13071-015-1033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emerson KJ, Conn JE, Bergo ES, Randel MA, Sallum MAM. Brazilian Anopheles darlingi root (Diptera: Culicidae) clusters by major biogeographical region. Plos One. 2015;10:1–15. doi: 10.1371/journal.pone.0130773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos M, et al. Microgeographical structure in the major Neotropical malaria vector Anopheles darlingi using microsatellites and SNP markers. Parasit. Vectors. 2017;10:76. doi: 10.1186/s13071-017-2014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lainhart W, et al. Evidence for temporal population replacement and the signature of ecological adaptation in a major Neotropical malaria vector in Amazonian Peru. Malar. J. 2015;14:375. doi: 10.1186/s12936-015-0863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armbruster P, Conn JE. Geographic Variation of Larval Growth in North American Aedes albopictus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2006;99:1234–1243. doi: 10.1603/0013-8746(2006)99[1234:GVOLGI]2.0.CO;2. [DOI] [Google Scholar]
- 16.Moller-Jacobs LL, Murdock CC, Thomas MB. Capacity of mosquitoes to transmit malaria depends on larval environment. Parasit. Vectors. 2014;7:593. doi: 10.1186/s13071-014-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro LLM, Whitehead SA, Thomas MB. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017;15:1–21. doi: 10.1371/journal.pbio.2003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro LLM, Murdock CC, Jacobs GR, Thomas RJ, Thomas MB. Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proc. R. Soc. B Biol. Sci. 2016;283:20160298. doi: 10.1098/rspb.2016.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beerntsen BT, James AA, Christensen BM. Genetics of Mosquito Vector Competence. Microbiol. Mol. Biol. Rev. 2000;64:115–37. doi: 10.1128/MMBR.64.1.115-137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdock CC, Sternberg ED, Thomas MB. Malaria transmission potential could be reduced with current and future climate change. Sci. Rep. 2016;6:27771. doi: 10.1038/srep27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt PS, Paaby AB. Reproductive Diapause and Life-History Clines in North American Populations of Drosophila melanogaster. Evolution (N. Y). 2008;62:1204–1215. doi: 10.1111/j.1558-5646.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- 22.Shelomi M. Where are we now? Bergmann’s rule sensu lato in insects. Am. Nat. 2012;180:511–9. doi: 10.1086/667595. [DOI] [PubMed] [Google Scholar]
- 23.Tang J, He H, Chen C, Fu S, Xue F. Latitudinal cogradient variation of development time and growth rate and a negative latitudinal body weight cline in a widely distributed cabbage beetle. Plos One. 2017;12:1–15. doi: 10.1371/journal.pone.0181030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demari-Silva B, Suesdek L, Sallum MAM, Marrelli MT. Wing geometry of Culex coronator (Diptera: Culicidae) from South and Southeast Brazil. Parasit. Vectors. 2014;7:174. doi: 10.1186/1756-3305-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barton M, Sunnucks P, Norgate M, Murray N, Kearney M. Co-gradient variation in growth rate and development time of a broadly distributed butterfly. Plos One. 2014;9:1–8. doi: 10.1371/journal.pone.0095258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlichting CD. The evolution of phenotypic plasticity in plants. Ann Rev Ecol Syst 17667–693. 1986;17:667–693. doi: 10.1146/annurev.es.17.110186.003315. [DOI] [Google Scholar]
- 27.Petino Zappala MA, Ortiz VE, Fanara JJ. Study of Natural Genetic Variation in Early Fitness Traits Reveals Decoupling Between Larval and Pupal Developmental Time in Drosophila melanogaster. Evol. Biol. 2018;45:437–448. doi: 10.1007/s11692-018-9461-z. [DOI] [Google Scholar]
- 28.Lefèvre T, et al. Beyond nature and nurture: Phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am. J. Trop. Med. Hyg. 2009;81:1023–1029. doi: 10.4269/ajtmh.2009.09-0124. [DOI] [PubMed] [Google Scholar]
- 29.Gimonneau G, et al. A behavioral mechanism underlying ecological divergence in the malaria mosquito Anopheles gambiae. Behav. Ecol. 2010;21:1087–1092. doi: 10.1093/beheco/arq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altamiranda M, Arboleda S, Parra JL, Peterson AT. Potential distribution of mosquito vector species in a primary malaria endemic region of Colombia. Plos One. 2017;12:e0179093. doi: 10.1371/journal.pone.0179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez GF, Correa MM. Discrimination of Neotropical Anopheles species based on molecular and wing geometric morphometric traits. Infect. Genet. Evol. 2017;54:379–386. doi: 10.1016/j.meegid.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Dantas C, et al. Multiple Blood Meals in Anopheles darlingi (Diptera: Culicidae) J. Vector Ecol. 2012;37:351–358. doi: 10.1111/j.1948-7134.2012.00238.x. [DOI] [PubMed] [Google Scholar]
- 33.Schilthuizen M, Kellermann V. Contemporary climate change and terrestrial invertebrates: Evolutionary versus plastic changes. Evol. Appl. 2014;7:56–67. doi: 10.1111/eva.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson D. Effects of temperature on the size of aquatic ectotherms: Exceptions to the general rule. J. Therm. Biol. 1995;20:61–74. doi: 10.1016/0306-4565(94)00028-H. [DOI] [Google Scholar]
- 35.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am. J. Trop. Med. Hyg. 2006;74:772–778. doi: 10.4269/ajtmh.2006.74.772. [DOI] [PubMed] [Google Scholar]
- 36.Hidalgo, K. et al. Seasonal variation in wing size and shape between geographic populations of the malaria vector, Anopheles coluzzii in Burkina Faso (West Africa). Acta Trop. 143 (2015). [DOI] [PubMed]
- 37.Lyimo EO, Takken W, Koella JC. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomol. Exp. Appl. 1992;63:265–271. doi: 10.1111/j.1570-7458.1992.tb01583.x. [DOI] [Google Scholar]
- 38.IPCC, 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, 10.1017/CBO9781107415324 (2013).
- 39.Olson SH, Gangnon RE, Silveira GA, Patz JA. Deforestation and malaria in Mâncio Lima county, Brazil. Emerg. Infect. Dis. 2010;16:1108–1115. doi: 10.3201/eid1607.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck-Johnson, L. M. et al. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. Plos One8 (2013). [DOI] [PMC free article] [PubMed]
- 41.Mordecai EA, et al. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett. 2013;16:22–30. doi: 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- 42.Lefèvre, T., Vantaux, A., Dabire, K. R., Mouline, K. & Cohuet, A. Non-Genetic Determinants of Mosquito Competence for Malaria Parasites. Plos Pathog. 9 (2013). [DOI] [PMC free article] [PubMed]
- 43.Hiwat H, Bretas G. Ecology of Anopheles darlingi Root with Respect to Vector Importance: A Review. Parasit. Vectors. 2011;4:1–13. doi: 10.1186/1756-3305-4-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendis K, et al. From malaria control to eradication: The WHO perspective. Trop. Med. Int. Heal. 2009;14:802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- 45.Protopopoff N, et al. Ranking malaria risk factors to guide malaria control efforts in African highlands. Plos One. 2009;4:1–10. doi: 10.1371/journal.pone.0008022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golding N, et al. Integrating vector control across diseases. BMC Med. 2015;13:1–6. doi: 10.1186/s12916-015-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benelli G, Mehlhorn H. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol. Res. 2016;115:1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 48.Brady OJ, et al. Vectorial capacity and vector control: Reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 2016;110:107–117. doi: 10.1093/trstmh/trv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grech K, Maung LA, Read AF. The effect of parental rearing conditions on offspring life history in Anopheles stephensi. Malar. J. 2007;6:130. doi: 10.1186/1475-2875-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phasomkusolsil S, et al. The relationship between wing length, blood meal volume, and fecundity for seven colonies of Anopheles species housed at the Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand. Acta Trop. 2015;152:220–227. doi: 10.1016/j.actatropica.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Barreaux, A. M. G., Barreaux, P., Thievent, K. & Koella, J. C. Larval environment influences vector competence of the malaria mosquito Anopheles gambiae. MalariaWorld J. 7 (2016). [DOI] [PMC free article] [PubMed]
- 52.Christiansen-Jucht CD, Parham PE, Saddler A, Koella JC, Basáñez M-G. Larval and adult environmental temperatures influence the adult reproductive traits of Anopheles gambiae s.s. Parasit. Vectors. 2015;8:456. doi: 10.1186/s13071-015-1053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. Deforestation and vectorial capacity of Anopheles gambiae giles mosquitoes in malaria transmission, Kenya. Emerg. Infect. Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forsman A. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity (Edinb). 2015;115:276–284. doi: 10.1038/hdy.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owusu HF, Chitnis N, Müller P. Insecticide susceptibility of Anopheles mosquitoes changes in response to variations in the larval environment. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-03918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bayoh MN, Lindsay SW. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med. Vet. Entomol. 2004;18:174–179. doi: 10.1111/j.0269-283X.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 57.Oliver SV, Brooke BD. The effect of larval nutritional deprivation on the life history and DDT resistance phenotype in laboratory strains of the malaria vector Anopheles arabiensis. Malar. J. 2013;12:44. doi: 10.1186/1475-2875-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christiansen-Jucht C, Parham PE, Saddler A, Koella JC, Basáñez MG. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae s.s. Parasites and Vectors. 2014;7:1–10. doi: 10.1186/s13071-014-0489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vittor A, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006;74:3–11. doi: 10.4269/ajtmh.2006.74.3. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira-Ferreira J, et al. Malaria in Brazil: an overview. Malar. J. 2010;9:115. doi: 10.1186/1475-2875-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barreaux AMG, Stone CM, Barreaux P, Koella JC. The relationship between size and longevity of the malaria vector Anopheles gambiae (s.s.) depends on the larval environment. Parasit. Vectors. 2018;11:485. doi: 10.1186/s13071-018-3058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couret, J., Dotson, E. & Benedict, M. Q. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). Plos One9 (2014). [DOI] [PMC free article] [PubMed]
- 63.Murdock CC, Blanford S, Luckhart S, Thomas MB. Ambient temperature and dietary supplementation interact to shape mosquito vector competence for malaria. J Insect Physiol. 2014;67:37–44. doi: 10.1016/j.jinsphys.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paaijmans KP, et al. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beck-Johnson, L. M. et al. The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk Subject Areas. R. Soc. Open Sci. 4 (2017). [DOI] [PMC free article] [PubMed]
- 66.Benedict MQ, Hood-Nowotny RC, Howell PI, Wilkins EE. Methylparaben in Anopheles gambiae s.l. sugar meals increases longevity and malaria oocyst abundance but is not a preferred diet. J. Insect Physiol. 2009;55:197–204. doi: 10.1016/j.jinsphys.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Lehmann T, et al. Genetic contribution to variation in larval development time, adult size, and longevity of starved adults of Anopheles gambiae. Infect. Genet. Evol. 2006;6:410–416. doi: 10.1016/j.meegid.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Moreno M, et al. Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am. J. Trop. Med. Hyg. 2014;90:612–616. doi: 10.4269/ajtmh.13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreno M, et al. Intensive trapping of blood-fed Anopheles darlingi in Amazonian Peru reveals unexpectedly high proportions of avian blood-meals. Plos Negl. Trop. Dis. 2017;11:1–19. doi: 10.1371/journal.pntd.0005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linthicum K. A Revision of the Argyritarsis Section of the Nyssorhynchus of Anopheles (Diptera: Culicidae) Mosq. Syst. 1988;20:101–271. [Google Scholar]
- 71.Motoki MT, Suesdek L, Bergo ES, Sallum MAM. Wing geometry of Anopheles darlingi Root (Diptera: Culicidae) in five major Brazilian ecoregions. Infect. Genet. Evol. 2012;12:1246–1252. doi: 10.1016/j.meegid.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Siminski A, Fantini AC, Guries RP, Ruschel AR, dos Reis MS. Secondary Forest Succession in the Mata Atlantica, Brazil: Floristic and Phytosociological Trends. ISRN Ecol. 2011;2011:1–19. doi: 10.5402/2011/759893. [DOI] [Google Scholar]
- 73.Rubio-Palis Y, Zimmerman RH. Ecoregional Classification of Malaria Vectors in the Neotropics. J. Med. Entomol. 1997;34:499–510. doi: 10.1093/jmedent/34.5.499. [DOI] [PubMed] [Google Scholar]
- 74.Eliceiri K, Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis Historical commentary NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth1012-1031b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carron A. Correlation between wing measurements and dry body weight in male and female Ochlerotatus (Ochlerotatus) caspius (Pallas, 1771)(Diptera: Culicidae) Eur. Mosq. Bull. 2007;24:4–8. [Google Scholar]
- 76.Armbruster P, Hutchinson RA. Pupal Mass and Wing Length as Indicators of Fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae) J. Med. Entomol. 2002;39:699–704. doi: 10.1603/0022-2585-39.4.699. [DOI] [PubMed] [Google Scholar]
- 77.Team, R. C. R: A language and environment for statistical computing. (2018).
- 78.Bates DM, Machler M, Bolker BM, Walker SC. Fitting Linear Mixed-Effects Models Using {lme4} J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 79.Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S. (Springer NY, 2002).
- 80.Therneau, T. M. A Package for Survival Analysis in S. (2015).
- 81.Kosinski, A. K. And M. survminer: Drawing Survival Curves using ‘ggplot2’. (2017).
- 82.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 83.IBGE. Brazilian Institute of Geography and Statistics (IBGE). Available at, https://www.ibge.gov.br/geociencias-novoportal/downloads-geociencias.html.
- 84.INMET. National Institute of Meteorology. Available at, http://www.inmet.gov.br/portal/.
- 85.SEDAM. Government of the State of Rondônia State Secretariat for Environmental Development. Available at, http://www.sedam.ro.gov.br/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its Supporting Information Files.