Fig. 4. Proteolytic stability and membrane permeability of PMIBcr/Abl-R6.
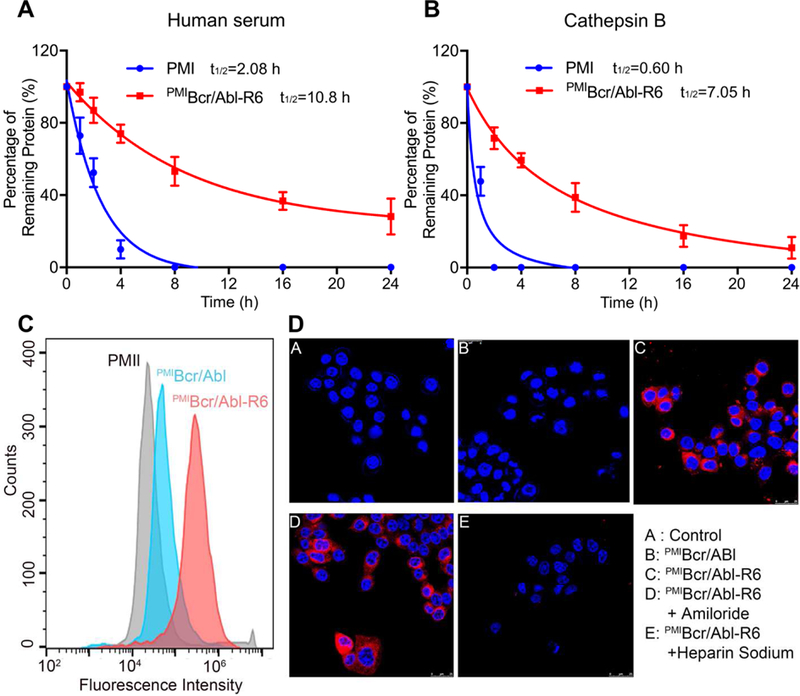
(A) Degradation kinetics of PMI and PMIBcr/Abl-R6 at 1 mg/ml in 20 mM Tris-HCl, pH 7.4, containing 10% human serum from a healthy donator. Intact peptide and protein were verified by ESI-MS and quantified by analytical C18 HPLC. (B) Degradation kinetics of PMI and PMIBcr/Abl-R6 at 1 mg/ml in 20 mM sodium acetate buffer, pH 5.0, containing cathepsin B at 10 units/ml. (C) Cellular uptake of PMI, PMIBcr/Abl and PMIBcr/Abl-R6 analyzed by flow cytometry. PMI, PMIBcr/Abl and PMIBcr/Abl-R6 were N-terminally labeled with BDP TR (excitation 589 nm, emission 616 nm). HCT 116 p53+/+ cells were seeded in a 12-well plate at a density of 30,000 cells/well, cultured for 24 h, and treated with peptide or protein at 10 µM for 4 h before flow cytometric analysis. (D) Cellular uptake of BDP TR-labeled PMI, PMIBcr/Abl and PMIBcr/Abl-R6 by HCT 116 p53+/+ cells, treated by peptide or protein at 10 µM each for 4 h, and visualized by a confocal laser scanning microscope (panels A-C). Hoechst 33342 blue dye was used for nuclei staining. For the experiments presented in panels D-E, amiloride (3 mM) or heparin sodium (5 mM) was incubated with cells for 12 h before the addition of PMIBcr/Abl-R6.
