Fig. 8. Safety evaluation of PMIBcr/Abl-R6 in vivo.
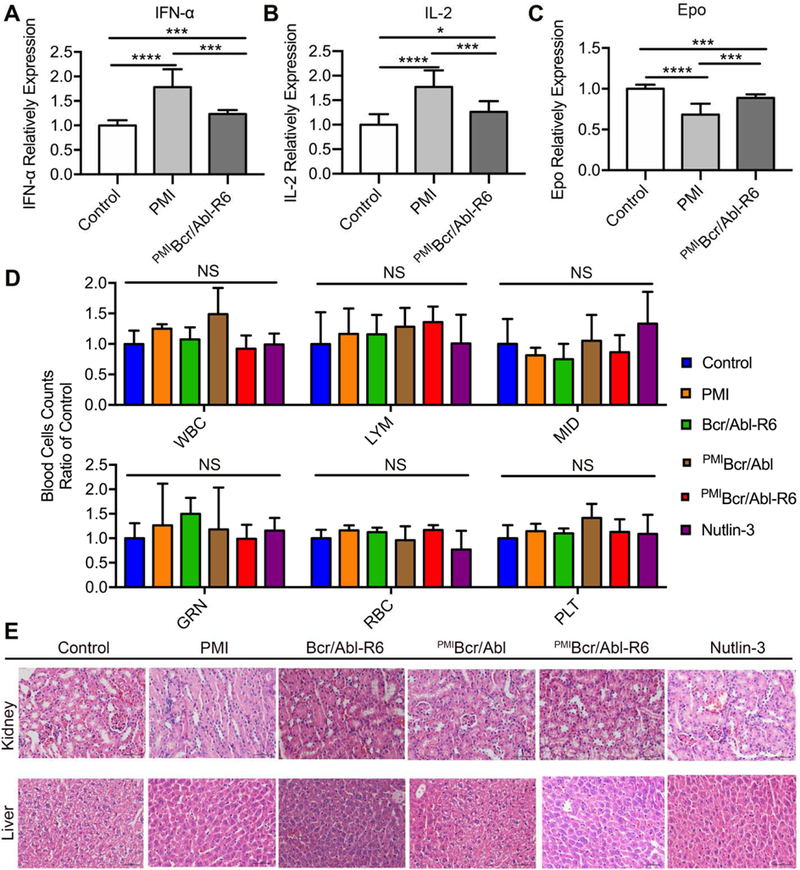
(A-C) Immunogenicity of PMI and PMIBcr/Abl-R6 in immune-competent C57BL/6 mice (n=6/group) as measured by the level of IL-2 (A), TNF-α (B) and erythropoietin (EPO) (C) in the blood in response to subcutaneous treatments with PMI and PMIBcr/Abl-R6 for three weeks, every other day, at a dose of 5 mg/Kg. PBS was used as a negative control for mock treatment; IL-2, TNF-α and EPO in the blood collected at the end of the treatment were quantified by ELISA kits (R&D Systems) using protein standards from Sigma-Aldrich. The data from each group are presented as the mean ± SD (n=6), and statistical analysis was performed using T-test, * standing for p<0.05, *** for p<0.001, and **** for p<0.0001. (D) Counts of different types of blood cells from a complete blood cell analysis after the 21-day treatment with PMI, Bcr/Abl-R6, PMIBcr/Abl, PMIBcr/Abl-R6 and Nutlin-3. WBC, white blood cell; LYM, lymphocyte; MID, monocyte; GRN, granulocytes; RBC, red blood cell; PLT, platelet. Statistical analysis was performed using T-test, NS standing for no significant difference. (E) Representative H&E staining of liver and kidney tissues from mice treated with with PMI, Bcr/Abl-R6, PMIBcr/Abl, PMIBcr/Abl-R6 and Nutlin-3 for three weeks (scale bar: 50 µm).
