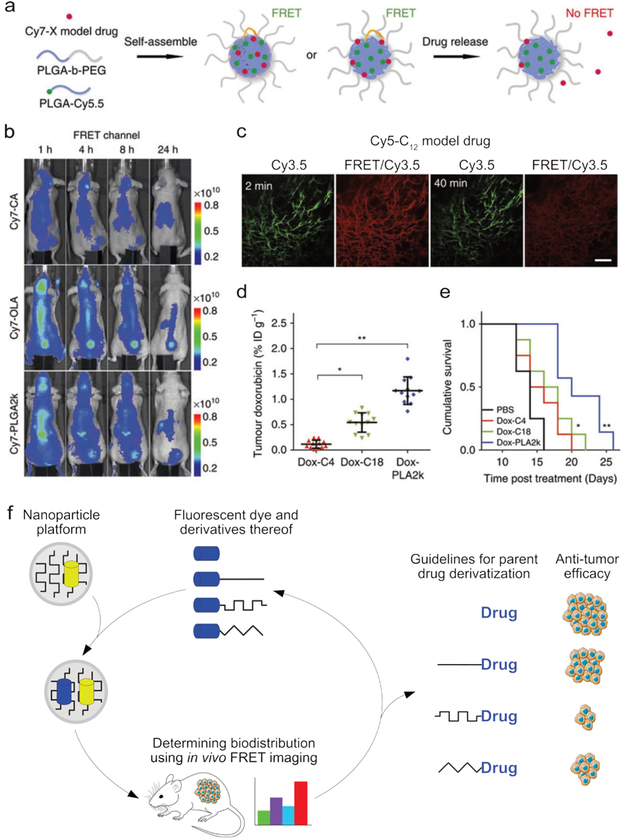
Fig. 8.
The in vivo stability and distribution of a supramolecular construct can be monitored using FRET. (a) The components of a polymeric nanoparticle were labeled with a FRET donor (Cy5.5), and a FRET acceptor (Cy7) was incorporated as a model drug. By measuring the decrease in FRET efficiency, the release of the model drug could be monitored. (b) Three formulations of the particles described in a were formed, each containing different derivatives of the Cy7 model drug, CA = carboxylic acid, OLA = oleylamine, PLGA = poly(lactic-co-glycolic acid). The biodistribution and structural integrity of the nanotherapeutic (i.e., the combined nanoparticle+model drug), could be visualized using FRET imaging. (c) Fluorescence images of a window chamber mouse injected with nanoparticles containing the Cy5-C12 model drug and Cy3.5 fluorophore, 2 and 40 minutes post injection. Scale bar = 100 μm injection (Cy3.5 was used instead of Cy7 to accommodate the microscopy protocol). (d) The tumor accumulation of doxorubicin observed in mice 24 hours after injection with nanoparticles containing one of three different doxorubicin derivatives, showing the strong influence of drug functionalization. (e) The cumulative survival curves corresponding to the experiments in d. Images a-e adapted with permission from ref12. Copyright 2016, Nature Publishing Group. (f) The biodistribution of a nanotherapeutic depends on the carrier’s size, composition, and compatibility with the incorporated drug. By labeling the carrier with a fluorophore and including a fluorescent model drug, the carrier’s stability and biodistribution can be monitored using FRET. In this way, the drug-carrier compatibility can be optimized, thereby enhancing nanotherapeutic efficacy.