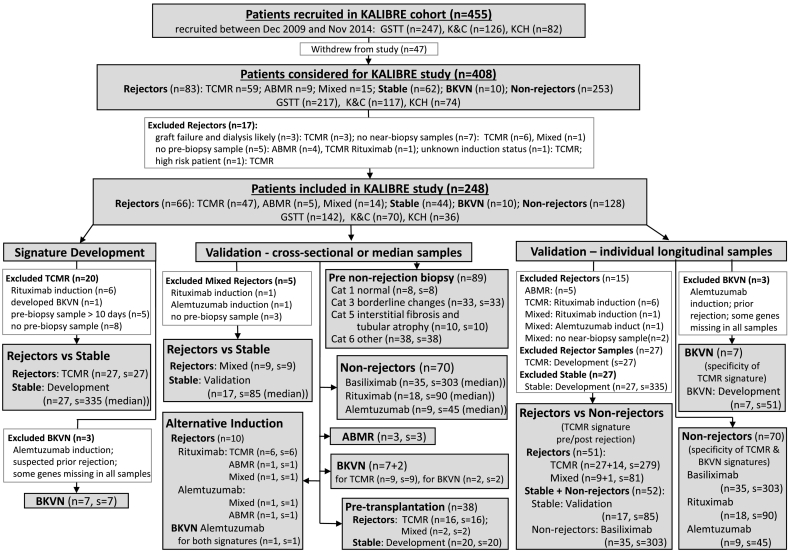
Fig. 1.
Flow diagram of patients from the KALIBRE study.
Biopsy categories were defined according to Banff ‘09 classification: GSTT – Guy's and St Thomas' NHS Foundation Trust (London, UK); KCH – King's College Hospital NHS Foundation Trust (London, UK); K&C – East Kent Hospitals University NHS Foundation Trust (Canterbury, UK); Cat – Banff category; ABMR – antibody-mediated rejection (category 2); TCMR – T-cell-mediated rejection (category 4); Mixed – histological features of both, ABMR and TCMR; BKVN – BK virus nephropathy (confirmed with a specialised histological staining); unless specifically indicated, patients received Basiliximab induction; Inclusion and exclusion criteria for patients in categories Rejector, Stable and BKVN for the discovery dataset are listed in Table 1; n – number of patients; s – number of samples; median – the median predicted probability from all samples of the same stable patient was used as representative for each stable patient in the signature development and cross-sectional validation.