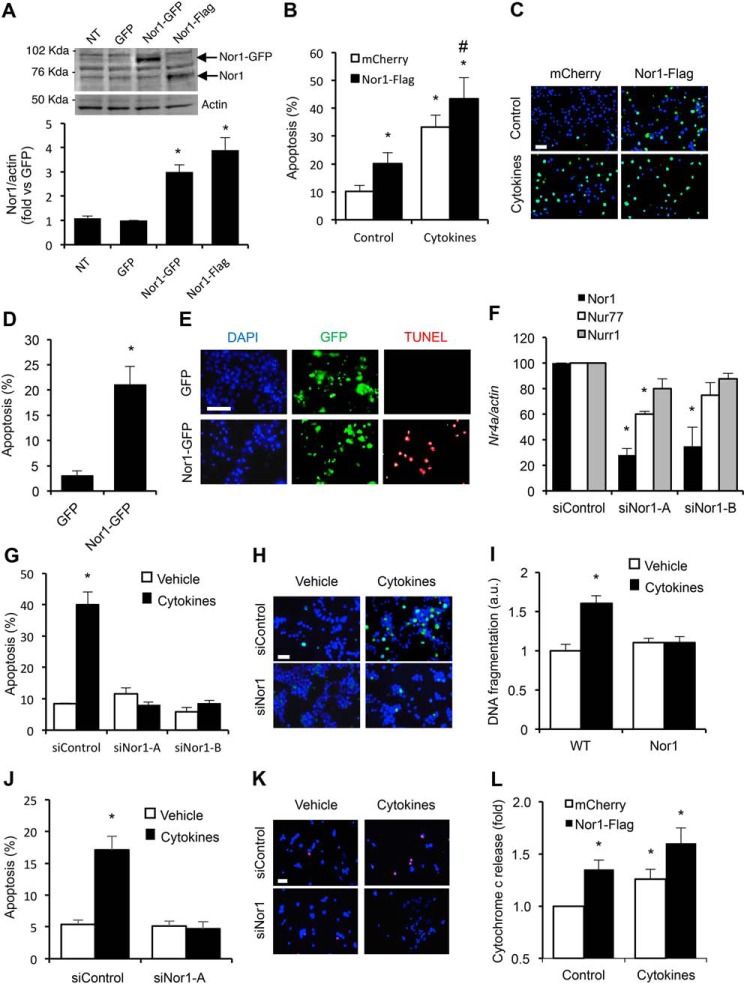
Figure 5.
Nor1 plays a critical role in INS and islet cell apoptosis. A, Nor1 protein levels were evaluated in nontransfected (NT) INS832/13 cells or 24 h after transfection with a control GFP plasmid, Nor1-GFP, or Nor1-FLAG (n = 5). A representative immunoblots is shown. Quantification results are represented as means ± S.E.; *, p < 0.05 versus control GFP. The arrows indicate the predicted Nor1 bands, with Nor1-GFP migrating at a higher molecular weight. B and C, apoptosis was assessed by TUNEL assay in INS832/13 cells transfected with Nor1 (black columns) or a control vector (white columns) and subsequently incubated in the presence or absence of cytokines for 24 h (n = 3). D and E, apoptosis was measured by TUNEL assay in dispersed human islets 24 h after transfection with Nor1-GFP or GFP alone. The results are represented as the percentages of TUNEL-labeled nuclei in the population of GFP-positive cells (n = 3). DAPI, 4′,6-diamidino-2-phenylindole. F, Nor1, Nur77, and Nurr1 expression was evaluated by qPCR in INS832/13 cells 48 h after transfection with a control siRNA or two different Nor1-specific siRNA sequences (n = 3). G and H, siRNA-mediated knockdown of Nor1 prevented cytokine-induced apoptosis. Apoptosis was determined by TUNEL in INS832/13 cells transfected with control or Nor1-specific siRNAs and incubated in the presence (black columns) or absence (white columns) of cytokines as described above (n = 3). I, the effects of cytokines on islet cell apoptosis was also investigated in intact WT and Nor1-KO mouse islets ex vivo by measuring the fragmentation of DNA using an ELISA assay (n = 5). J and K, siRNA-mediated knockdown of Nor1 prevented cytokine-induced apoptosis in human islet cells. L, Nor1 caused cytochrome c release. Cells were treated as described in B, fractionated into cytoplasmic and mitochondrial fractions, and analyzed by ELISA (n = 4). All results are represented as means ± S.E.; *, p < 0.05 versus control; # p < 0.05 versus PCMV-Nor1-FLAG without cytokines. Scale bars = 50 μm.