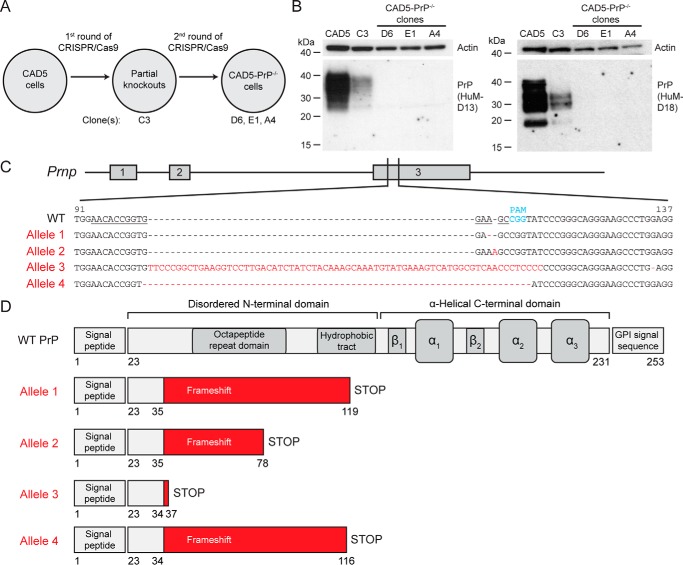
Figure 1.
Generation of CAD5-PrP−/− cells. A, schematic of the procedure used to isolate CAD5-PrP−/− cells. Four monoclonal lines were kept for further analysis: The partial knockout clone C3 as well as CAD5-PrP−/− clones D6, E1, and A4. B, immunoblots of PrP levels in cell lysates from WT CAD5 cells, the partial knockout clone C3, and the CAD5-PrP−/− lines D6, E1, and A4. PrP was detected using the antibodies HuM-D13 (left blot) or HuM-D18 (right blot). The lower molecular weight PrP bands visible with the HuM-D18 antibody represent a physiological cleavage product termed C1 which is generated by endoproteolysis in the vicinity of PrP residue 110 (74). Blots were reprobed with an antibody to actin (20–33) to compare total protein levels. Molecular mass measurements are indicated in kDa. C, characterization of mutant Prnp alleles in CAD5-PrP−/− clone D6. The numbering is with respect to the ORF of WT Prnp. The targeting sequence of the gRNA is underlined, and the protospacer-adjacent motif (PAM) is indicated in blue. Four mutant alleles were identified: An adenine deletion (Allele 1), an adenine insertion (Allele 2), a large insertion (Allele 3), and a 10-bp deletion (Allele 4). The mutated residues present in the mutant alleles are indicated in red. D, schematic of truncated protein products predicted to be produced by the four mutant alleles. Beginning at PrP residues 34–35, frameshift mutations result in the incorporation of various amounts of non-PrP sequence (red) as well as a premature STOP codon. The domain structure of WT PrPC is shown for comparison.