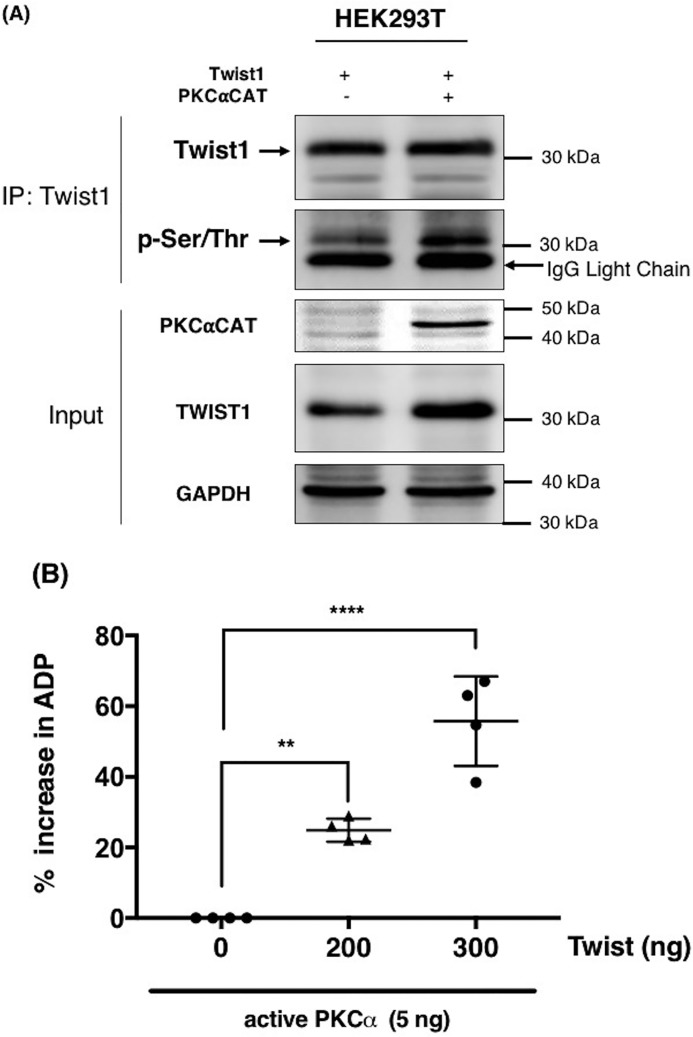
Figure 2.
PKCα induces Twist1 phosphorylation. A, Twist1 was transfected in HEK293T cells in the presence or absence of constitutively active PKCαCAT. Empty vector was used as control. Twist1 was subsequently immunoprecipitated, and the levels of phosphorylated Twist1 were determined by Western blotting using anti–phospho-serine/tyrosine/threonine antibody. GAPDH was used as a loading control for the input. B, in vitro kinase assay was performed as described under “Experimental procedures” with recombinant PKCα in the presence of increasing concentration of recombinant Twist1 as substrate. The ability of PKCα to phosphorylate Twist1 was quantified by the amount of ADP produced and presented as percentages of increase in ADP. *, p = 0.0022; **, p = 0.0001, compared with kinase reaction in the absence of Twist1. The experiments were performed at least three independent times. Representative data are shown.