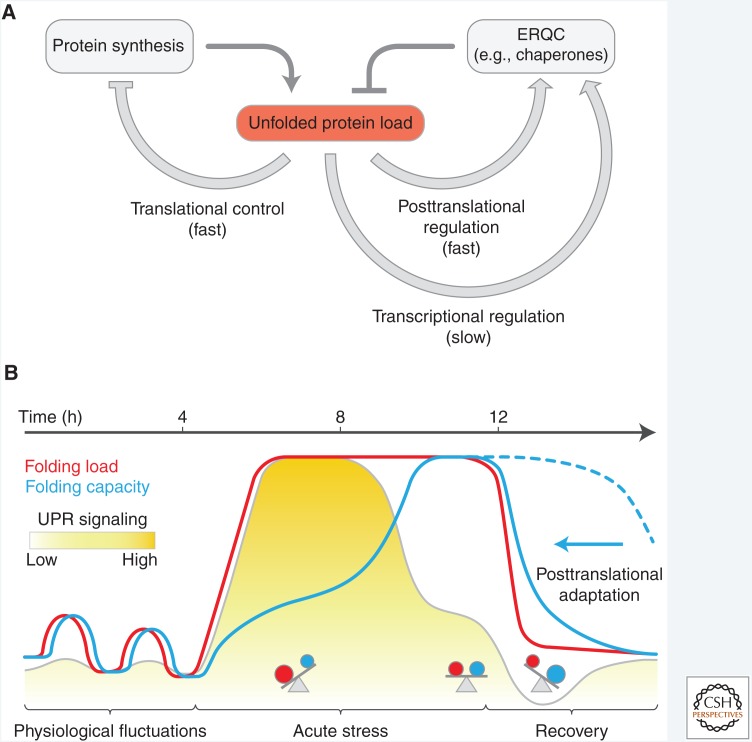
Figure 2.
Latency of the negative feedback-regulated transcriptional unfolded protein response (UPR). (A) Adaptation of the UPR by negative feedback regulation via endoplasmic reticulum (ER) protein quality control (ERQC) components. Apart from stress-denatured proteins, newly synthesized secretory proteins constitute the majority of unfolded species in the ER and their accumulation activates the UPR transducers. The response entails rapid attenuation of translation to alleviate the unfolded protein load (see Fig. 5), rapid posttranslational regulation of the pool of active chaperones in the ER (see Fig. 3), and the slower induction of ERQC genes to expand the folding capacity and mediate negative feedback regulation of the UPR (see Fig. 4). (B) Theoretical, time-resolved response profiles to illustrate the inherent latency of the UPR. Recurring physiological short-term fluctuations in the unfolded protein load do not cause a significant transcriptional response, suggesting that they are buffered by rapid, nontranscriptional adjustments of the folding capacity. During acute stress the fast rise of the folding load induces UPR signaling but expansion of the folding capacity is delayed as a result of the inherent latency of transcriptional reprogramming. Negative feedback inhibition attenuates the transcriptional response as the folding capacity builds up to establish homeostasis at a new level. During the recovery from ER stress the folding load drops rapidly. Were the adjustment in ERQC capacity to rely exclusively on the decay of the ERQC messenger RNAs (mRNAs) and encoded proteins, ERQC capacity would likely exceed the unfolded protein load in this recovery phase (dotted blue line). However, posttranslational mechanisms that inactivate chaperones rapidly adjust the effective folding capacity and prevent over-chaperoning (arrow).