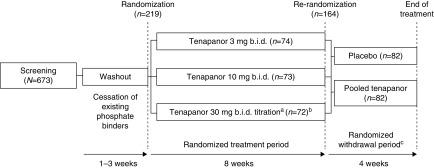
Figure 1.
Study design. aPatients initially receiving tenapanor 30 mg twice a day were allowed to down-titrate weekly (stepwise 30 → 20 → 15 → 10 → 3 mg twice a day) during the first 4 weeks of the RTP, on the basis of gastrointestinal tolerability. Mean final dose of tenapanor in this group at the end of the RTP was 24.4 mg twice a day for the safety and ITT analysis sets, and 22.8 mg twice a day for the secondary efficacy analysis set. bOne patient did not receive any dose of study drug and was excluded from analyses. cThe study was initiated on January 20, 2016 as an 8-week randomized dose range–finding study; the RWP was added to the protocol in an amendment dated March 3, 2016, when 22 patients were enrolled. Patients randomized to tenapanor in the RWP remained on their previous dose from the RTP. Mean exposure during the RTP was 48 days in all three treatment groups, and during the RWP was 26 days for patients receiving placebo and 27 days for patients receiving tenapanor. bid, twice daily.